Aaron Aponick of the University of Florida achieved high ee in the
cyclization of the allylic alcohol 1 to the
lactone 2
(Angew. Chem. Int. Ed. 2021, 60, 22224.
DOI: 10.1002/anie.202108336).
Wanxiang Zhao of Hunan University also achieved high facial selectivity in the hydroboration of the enol
ether 3, leading to the secondary alcohol 4
(J. Am. Chem. Soc. 2021, 143, 10902.
DOI: 10.1021/jacs.1c06697).
Xiaohua Liu and Xiaoming Feng of Sichuan University prepared the
epoxide 6 from the α-methylene ketone 5
(Org. Lett. 2-Hydroxy-5-(hydroxymethyl)benzaldehyde Price 2021, 23, 6961.
DOI: 10.1021/acs.orglett.1c02588).
Long Zhang and Sanzhong Luo of Tsinghua University effected the enantioselective
α-hydroxylation of the β-ketoester
7, leading to the tertiary alcohol 8
(J. Am. Chem. Soc. DMT-2’fluoro-da(bz) amidite Price 2021, 143, 1078.
DOI: 10.1021/jacs.0c11787).
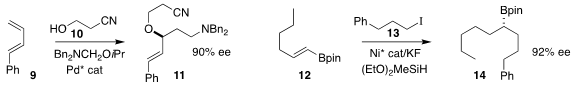
Hanmin Huang of the University of Science and Technology of China assembled
the ether 11 by adding the alcohol 10 to the diene 9
(J. Am. Chem. PMID:24456950 Soc. 2021, 143, 12467.
DOI: 10.1021/jacs.1c06144).
Xile Hu of the Ecole Polytechnique Fédérale de Lausanne prepared the secondary
alkyl
borane 14 by coupling the alkenyl borane 12 with the iodide 13
(Nature Chem. 2021, 13, 270.
DOI: 10.1038/s41557-020-00576-z).
James P. Morken of Boston College described a complementary approach
(J. Am. Chem. Soc. 2021, 143, 14189.
DOI: 10.1021/jacs.1c05274).
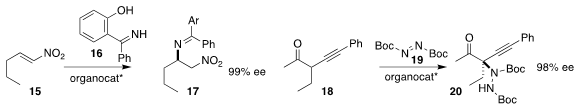
Alberto Fraile and José Alemán of the Universidad Autónoma de Madrid added
the imine 16 to the nitroalkene 15, leading to the
secondary amine 17
(Adv. Synth. Catal. 2021, 363, 3845.
DOI: 10.1002/adsc.202100635).
Houchao Tao and Xiaoyu Yang of ShanghaiTech University achieved high ee in the addition of
the α-alkynyl ketone 18 to the diazodicarboxylate 19, leading to the
protected hydrazine 20
(Org. Chem. Front. 2021, 8, 5377.
DOI: 10.1039/D1QO00720C).
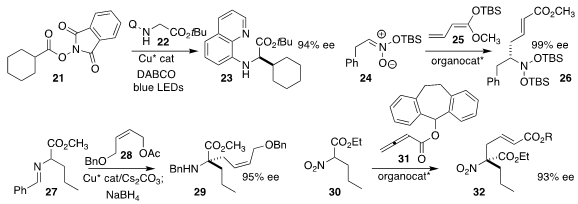
Rui Wang and Zhaoqing Xu of Lanzhou University used visible light to promote the coupling of
the N-hydroxyphthalimide ester 21 with the protected glycine 22,
to give the α-amino ester 23
(J. Am. Chem. Soc. 2021, 143, 12777.
DOI: 10.1021/jacs.1c05890).
Benjamin List of of the Max-Planck-Institut für Kohlenforschung prepared the secondary amine
26 by adding the ketene silyl acetal 25 to the silyl nitronate 24
(Nature Catal. 2021, 4, 1043.
DOI: 10.1038/s41929-021-00714-x).
Shu-Li You of the Shanghai Institute of Organic Chemistry assembled the α-quaternary
amine 29 by alkylating the α-amino ester 27 with the
allylic acetate 28, followed by reduction
(Science 2021, 371, 380.
DOI: 10.1126/science.abd609).
Yixin Lu of the National University of Singapore achieved high ee in the addition of the α-nitro
ester 30 to the allenoate 31, leading to the α-quaternary nitro ester 32
(Org. Chem. Front. 2021, 8, 6114.
DOI: 10.1039/D1QO01016F).
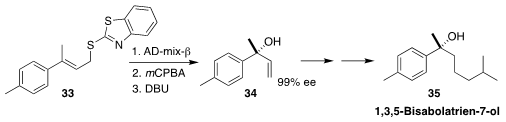
1,3,5-Bisabolatrien-7-ol (35) was isolated from the essential oil of mango
ginger Curcuma amada. Arata Yajima of the Tokyo University of Agriculture established
the absolute configuration of 35 by
Sharpless asymmetric dihydroxylation of the alkene
33, followed by elimination to give the delicate tertiary alcohol 34
(Tetrahedron 2021, 92, 132253.
DOI: 10.1016/j.tet.2021.132253).