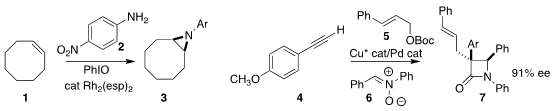
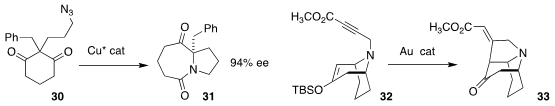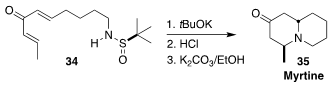Tom G. Driver of the University of Illinois at Chicago developed oxidative
conditions for the preparation of the
aziridine 3 by the addition of the aniline
2 to the alkene 1
(J. Am. PMID:25105126 Chem. Soc. 2021, 143, 19149.
DOI: 10.1021/jacs.1c09229).
Zhenghu Xu of Shandong University constructed the
β-lactam 7 by the three-component
coupling of the alkyne 4, the allylic carbonate 5, and the nitrone 6
(Angew. Chem. Int. Ed. 2-(Bromomethyl)-4-fluoro-1-nitrobenzene custom synthesis 2021, 60, 13814.
DOI: 10.1002/anie.202100601).
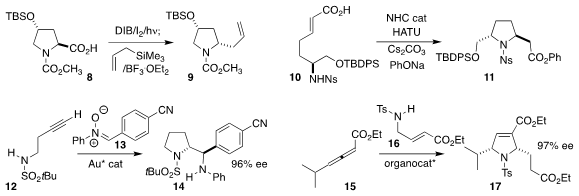
Dacil Hernández and Alicia Boto of the Instituto de Productos Naturales y
Agrobiología del CSIC oxidized the proline derivative 8, then coupled the
intermediate with allyl trimethylsilane to give the
pyrrolidine 9
(J. Org. Chem. Buy946000-13-1 2021, 86, 2796.
DOI: 10.1021/acs.joc.0c02751).
Chun-Lin Zhang and Song Ye of the Institute of Chemistry of the
Chinese Academy of Sciences observed high diastereoselectivity in the
cyclization of the unsaturated acid 10 to the sulfonamide 11
(Tetrahedron 2021, 94, 132337.
DOI: 10.1016/j.tet.2021.132337).
Xinfang Xu of Sun Yat-sen University achieved high diastereoselectivity and enantioselectivity
in the gold-catalyzed coupling of the alkyne 12 with the nitrone 13, to give 14
(Chem. Commun. 2021, 57, 12171.
DOI: 10.1039/D1CC04830A).
Liezhong Chen of the Zhejiang Academy of Agricultural Sciences and Hongchao Guo of China
Agricultural University used a tricyclic amino phosphine to catalyze the addition of
the amine 16 to the allene 15, leading to the
dihydropyrrole 17
(Org. Lett. 2021, 23, 9173.
DOI: 10.1021/acs.orglett.1c03483).
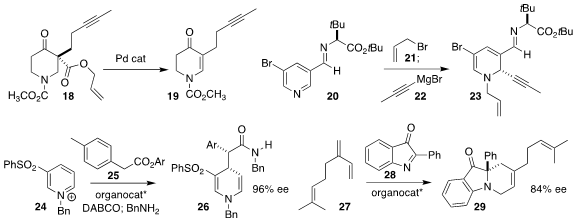
Alois Fürstner of the Max-Planck-Institut für Kohlenforschung used the Tsuji
protocol to prepare the enone 19 from the allyl α-ketoester 18
(J. Am. Chem. Soc. 2021, 143, 14402.
DOI: 10.1021/jacs.1c07955).
Joel M. Smith of Florida State University quaternized the
pyridine 20 with allyl
bromide (21), then observed high diastereoselectivity in the addition of the alkynyl
Grignard reagent 22, leading to the 1,2-dihydropyridine 23
(Org. Lett. 2021, 23, 6703.
DOI: 10.1021/acs.orglett.1c02276).
Andrew D. Smith of the University of St. Andrews assembled the
1,4-dihydropyridine 26 by the
isothiourea-mediated addition of the ester 25 to the pyridinium salt 24
(Chem. Sci. 2021, 12, 12001.
DOI: 10.1039/D1SC03860E).
Xin Li of Nankai University showed that a BINOL-derived phosphoric acid was an effective
catalyst for the aza-Diels-Alder combination of the diene 27 with the ketone 28,
leading to 29 with high regioselectivity
(Angew. Chem. Int. Ed. 2021, 60, 17608.
DOI: 10.1002/anie.202104788).
Fu-Min Zhang and Yong-Qiang Tu of Lanzhou University used a Cu catalyst to effect the
enantioselective ring expansion of the prochiral diketone 30 to the lactam 31
(Angew. Chem. Int. Ed. 2021, 60, 22688.
DOI: 10.1002/anie.202107909).
Hiroyuki Nakamura of the Tokyo Institute of Technology showed that a gold catalyst efficiently
cyclized the alkyne 32 to the tricyclic amine 33
(Chem. Eur. J. 2021, 27, 11888.
DOI: 10.1002/chem.202101440).
The alkaloid myrtine (35) was isolated from the European bilberry, Vaccinium
myrtillus. Carlos del Pozo of the University of Valencia developed a simple route
to 35, based on the diastereoselective double cyclization of the dienone 34
(Org. Biomol. Chem. 2021, 19, 8740.
DOI: 10.1039/D1OB01488A).
We note with sadness the passing of Professor Jiro Tsuji, who contributed so
much to our understanding of metal catalysis in organic synthesis.