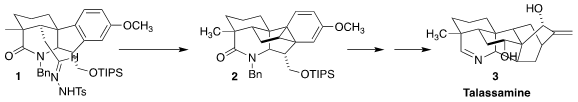
The structure of talassamine (3), isolated from the epigeal part of
Aconitum talassicum (Ranunculaceae), a perennial herb of Tibet and western
China, was established by x-ray analysis. En route to 3, Min Zhang of
Chongqing University cyclized the tosylhydrazone 1 to the hexacyclic
diene 2
(J. Am. 8-Bromo-3-chloroisoquinoline Chemical name Chem. Soc. PMID:24834360 2021, 143, 7088.
DOI: 10.1021/jacs.1c01865).
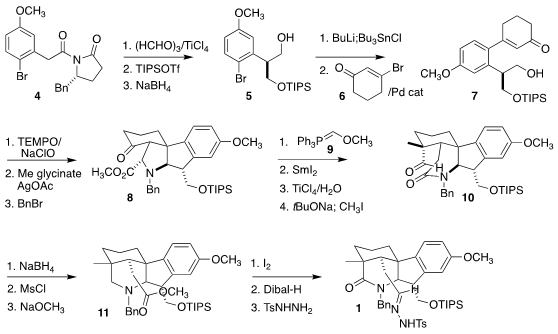
The preparation of 1 began with the acyl oxazolidinone 4. Evans
asymmetric aldol reaction followed by protection and reduction led to the
alcohol 5. The derived
aryl stannane was coupled with 3-bromocyclohexenone 6,
leading to the enone
7. Oxidation of the alcohol to the aldehyde and condensation with methyl
glycinate was followed by intramolecular
dipolar
cycloaddition, leading after
protection to the
amine 8. Formula of [Ir(dFppy)2(dtbbpy)]PF6 Reaction with the phosphorane 9 followed by reductive
cleavage of the C-N bond led to a
lactam, that was
deprotected and alkylated to give 10. Exposure of the derived mesylate to
NaOCH3 led via intramolecular N-alkylation to the ester 11.
Oxidation to the lactam followed by reduction to the aldehyde and condensation
with TsNHNH2 completed the assembly of the tosylhydrazone 1.
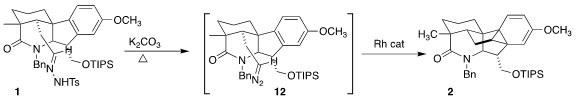
Heating with anhydrous K2CO3 converted the
tosylhydrazone 1 to the diazoalkane 12. This was long-lived enough
to combine with the Rh(II) catalyst to give an intermediate carbene, that added
to the aromatic ring to give the cyclopropane 2. The more soluble Rh2(octanoate)4
was more effective in this application than the less-soluble, oligomeric Rh2(acetate)4.
Reduction of the lactam to the amine was followed by acid-mediated cleavage
of the cyclopropane, that installed the requisite equatorial secondary hydroxyl
group. Hydrogenation followed by protection led to the ketone 13.
Desilylation was
followed by oxidation and intramolecular
aldol reaction to give
the mesylate 14. Exposure of the derived alcohol to the Tebbe reagent led
to 15. Dissolving metal removed the mesylate, after which allylic
oxidation followed by reduction, deprotection, and oxidation of the secondary
amine to the imine completed the synthesis of talassamine (3).
The approach outlined here to talassamine (3) underlines the strategic
power of in situ-generated alkyl and dialkyl diazo intermediates, pioneered by Aggarwal
(Angew. Chem. Int. Ed. 2001, 40, 1430,
DOI: 10.1002/1521-3773(20010417)40:8<1430::AID-ANIE1430>3.0.CO;2-W);
1433, DOI: 10.1002/1521-3773(20010417)40:8<1433::AID-ANIE1433>3.0.CO;2-E).
We can expect many more such applications.