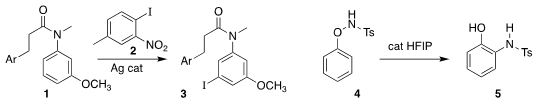
Shangda Li and Gang Li of the Fujian Institute of Research on the Structure
of Matter used the iodo nitro aromatic 2 to selectively
iodinate the anilide 1,
leading to 3
(Org. Lett. 2022, 24, 3657.
DOI: 10.1021/acs.orglett.2c01283).
Hui Gao, Zhi Zhou and Wei Yi of the
Guangzhou Medical University rearranged the phenoxysulfonamide 4 to the
o-sulfonamidophenol 5
(Tetrahedron Lett. 2022, 89, 153601.
DOI: 10.1016/j.tetlet.2021.153601). 105751-18-6 manufacturer
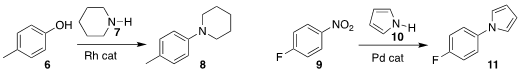
Hang Shi of Westlake University coupled the phenol 6 with piperidine
7 to give the aryl
amine 8
(J. Am. Chem. Soc. 2022, 144, 1144.
DOI: 10.1021/jacs.1c12622).
Lin Yu and Wengui Duan of Guangxi University coupled
pyrrole 10 with the nitro aromatic
9, leading to the protected aniline 11
(Org. Chem. 3-Butyn-1-ol supplier Front. 2022, 9, 2351.
DOI: 10.1039/D2QO00010E). PMID:35850484
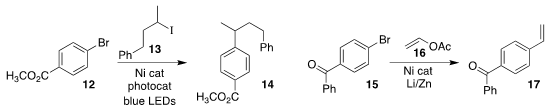
Veera Reddy Yatham of the Indian Institute of Science Education and Research
devised conditions for coupling the bromobenzene
12 with the secondary iodide
13, to give 14
(J. Org. Chem. 2022, 87, 5442.
DOI: 10.1021/acs.joc.2c00251).
Chuanhu Lei of Shanghai University and Jian Jin of the Shanghai Institute of Organic Chemistry prepared
the styrene derivative
17 by coupling vinyl acetate 16 with the bromobenzene 15
(Org. Lett. 2022, 24, 354.
DOI: 10.1021/acs.orglett.1c04018).
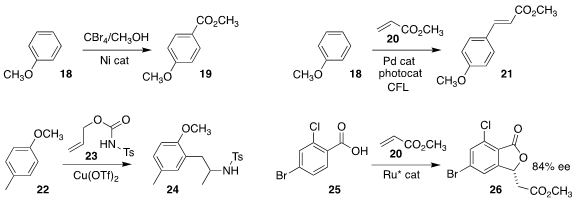
Shun-Jun Ji and Yingsheng Zhao of Soochow University established conditions
for adding a carboxyl group
para on anisole 18, to give 19
(Org. Lett. 2022, 24, 2155.
DOI: 10.1021/acs.orglett.2c00417).
Debabrata Maiti of the Indian Institute of Technology Bombay effected
the direct oxidative Heck coupling of anisole 18 with methyl acrylate 20,
leading to the ester 21
(J. Am. Chem. Soc. 2022, 144, 1929.
DOI: 10.1021/jacs.1c12311).
Giovanni Poli of Sorbonne Université and Gianluigi Broggini of the Università degli Studi
dellInsubria assembled the sulfonamide 24 by coupling 4-methyl anisole
22 with the O-allyl carbamate 23
(Org. Chem. Front. 2022, 9, 1711.
DOI: 10.1039/D2QO00114D).
Dattatraya H. Dethe of the Indian Institute of Technology Kanpur achieved significant enantiomeric
excess in the preparation of the lactone
26 by the coupling of the benzoic acid 25 with methyl acrylate 20
(J. Org. Chem. 2022, 87, 4617.
DOI: 10.1021/acs.joc.1c02961).
Haifeng Yu and Wenju Wang of Baicheng Normal University assembled the
benzamide 29 by adding nitroethane 28 to the ketene dithioacetal
27
(J. Org. Chem. 2022, 87, 2985.
DOI: 10.1021/acs.joc.1c02825).
Ravindar Kontham of CSIR-National Chemical Laboratory
showed that the intial adduct between the enone 30 and the alkyne 31 aromatized,
leading to the chromane 32
(Org. Chem. Front. 2022, 9, 802.
DOI: 10.1039/D1QO01643A).
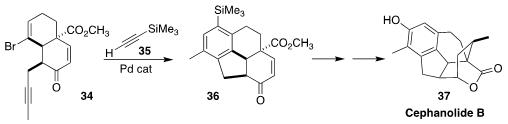
The cephanolide diterpenoids isolated from the coniferous shrub Cephalotaxus
sinensis, exemplified by cephanolide B (37), show antineoplastic, antiviral and
antitumor properties. Hongbin Zhai of the Shenzhen Graduate School of Peking
University established a route to cephanolides A-D, based on the construction of
the tetracyclic ketone 36 by the cyclization of the alkyne 34 with the alkyne
35
(J. Am. Chem. Soc. 2022, 144, 10640.
DOI: 10.1021/jacs.2c03978).