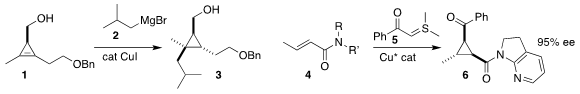
Ilan Marek of Technion – Israel Institute of Science and Technology
demonstrated that the homoallylic ether of the cyclopropene 1 was sufficient to
direct the regioselectivity of the addition of the Grignard reagent 2 to give
the cyclopropane 3
(Angew. Chem. Int. Ed. BuyPd-PEPPSI-IHept-Cl 2021, 60, 26368.
DOI: 10.1002/anie.202111382).
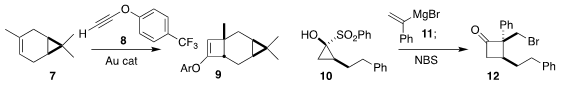
Naoya Kumagai and Masakatsu Shibasaki of BIKAKEN constructed the cyclopropane 6 by the
enantioselective addition of the sulfonium ylide 5 to the unsaturated amide 4
(ACS Catal. PMID:24257686 2021, 11, 11597.
DOI: 10.1021/acscatal.1c02723).
Antonio M. Echavarren of ICIQ used a gold catalyst to mediate the addition of
the alkynyl ether 8 to the alkene 7, leading to the
cyclobutanone precursor 10
(Org. (S)-2-Fluoropropanoic acid manufacturer Lett. 2021, 23, 8989.
DOI: 10.1021/acs.orglett.1c03499).
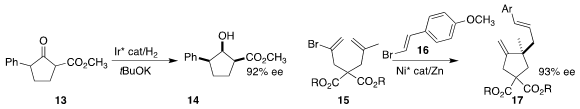
Vincent N. G. Lindsay of North Carolina State
University assembled the cyclobutanone 12 by adding the Grignard reagent
11 to the cyclopropanone
generated in situ from the cyclopropyl sulfone 10, then
brominating the product
(Org. Lett. 2021, 23, 6482.
DOI: 10.1021/acs.orglett.1c02303).
Jian-Hua Xie of Nankai University achieved high diastereoselectivity and enantioselectivity
in the reduction of the racemic ketone 13 to the hydroxy ester 14
(Org. Lett. 2021, 23, 5153,
DOI: 10.1021/acs.orglett.1c01689;
8883, DOI: 10.1021/acs.orglett.1c03384).
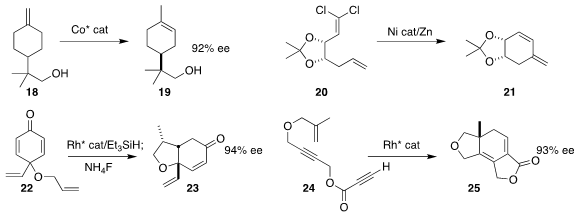
Xing-Zhong Shu of Lanzhou University
prepared the cyclopentane
17 by coupling the diene 15 with the bromoalkene 16
(J. Am. Chem. Soc. 2021, 143, 12961.
DOI: 10.1021/jacs.1c05670).
Qiang Liu of Tsinghua University used a Co catalyst to
isomerize the
prochiral alkene 18 to the alcohol 19
(J. Am. Chem. Soc. 2021, 143, 20633.
DOI: 10.1021/jacs.1c11343).
Christopher Uyeda of Purdue University reduced 20 to the intermediate vinylidene
(also termed “alkylidene”) carbene, that inserted into the proximal alkenyl C-H
bond to give the diene 21
(ACS Catal. 2021, 11, 14408.
DOI: 10.1021/acscatal.1c03350).
Wenbo Ye, Dingding Gao and Ping Tian of the Shanghai University of Traditional Chinese Medicine
cyclized the prochiral cyclohexadienone 22 to the
cyclohexenone 23
(Chem. Commun. 2021, 57, 9724.
DOI: 10.1039/D1CC03645A).
Takesi Yasui and Yoshihiko Yamamoto of Nagoya University cyclized
the propiolate 24 to the tricyclic lactone 25
(Adv. Synth. Catal. 2021, 363, 4182,
DOI: 10.1002/adsc.202100513;
ACS Catal. 2021, 11, 9479,
DOI: 10.1021/acscatal.1c02410).
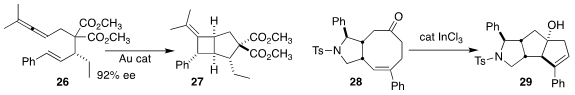
Guo-Qiang Lin and Zhi-Tao He of the Shanghai Institute of Organic Chemistry
developed a simple enantioselective route to the diester 26, that they subjected
to Toste cyclization to give the bicyclic 27
(Nature Commun. 2021, 12, 5626.
DOI: 10.1038/s41467-021-25978-6).
Zhi-Xiang Yu of Peking University devised conditions for the transannular
ene cyclization
of the cyclooctenone 28 to the tricyclic alcohol 29
(Org. Lett. 2021, 23, 7566.
DOI: 10.1021/acs.orglett.1c02766).
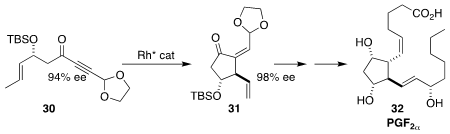
Gen-Qiang Chen and Xumu Zhang of the Southern University of Science and
Technology established an efficient route to the prostaglandins, exemplified by
PGF2α (32), based on the enantioselective cyclization of the
alkyne 30 to the diene 31
(Nature Chem. 2021, 13, 692.
DOI: 10.1038/s41557-021-00706-1).
The diene 31 should also allow ready access to the isoprostanes,
endogenous mediators of inflammatory damage.