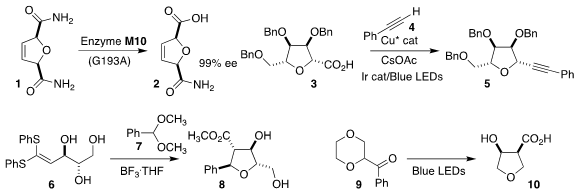
Yu-Fei Ao and Hui Chen of the Institute of Chemistry, Chinese Academy of
Sciences devised a mutant amidase that hydrolyzed the prochiral dihydrofuran bis
amide 1 to the monoacid 2 with high enantioselectivity
(ACS Catal. 2021, 11, 6900.
DOI: 10.1021/acscatal.1c01220).
Samir Messaoudi of the Université Paris-Saclay constructed the furanosyl alkyne 5
by coupling the tetrahydrofuranyl carboxylic acid 3 with the alkyne 4
(ACS Catal. 2021, 11, 6334.
DOI: 10.1021/acscatal.1c01600).
Tom D. Sheppard of University College London assembled the tetrahydrofuran 8
by combining the acetal 7 with the 1,1-bis(phenylthio)alkene 6
(Org. Lett. Pd 122 site 2021, 23, 2488.
DOI: 10.1021/acs.orglett.1c00424).
Yu-hong Lam and Charles S. PMID:24423657 Yeun of Merck and Richmond Sarpong of the University of California, Berkeley
developed the diastereoselective photochemical ring contraction of the acyl dioxane 9 to the
tetrahydrofuran 10
(Science 2021, 373, 1004.
DOI: 10.1126/science.abi7183). Rhodamine B isothiocyanate site
Xiang-Guo Hu of Jiangxi Normal University prepared the C-glycoside 13 by the photochemical cyclization
of the imidate 11 in the presence of styrene 12
(Org. Lett. 2021, 23, 2659.
DOI: 10.1021/acs.orglett.1c00551).
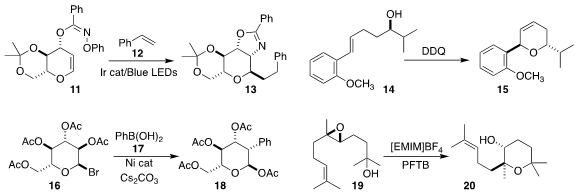
Dongjoo Lee of Ajou University devised the oxidative
cyclization of the alkenyl alcohol 14 to the dihydropyran 15
(Org. Lett. 2021, 23, 1135.
DOI: 10.1021/acs.orglett.1c00154).
Peng Liu of the University of Pittsburgh and Ming-Yu Ngai of the State University of New York,
Stony Brook prepared the C-2 arylated glucose 18 by coupling the glucosyl bromide
16 with phenylboronic acid 17
(J. Am. Chem. Soc. 2021, 143, 8590.
DOI: 10.1021/jacs.1c03563).
Jin Qu of Nankai University developed conditions to redirect the cyclization of the epoxide
19 from the expected exo product to the valuable endo product 20,
with clean inversion of absolute configuration
(Angew. Chem. Int. Ed. 2020, 59, 18473.
DOI: 10.1002/anie.202007980).
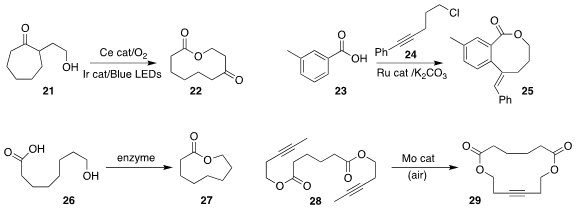
Zhiwei Zuo of ShanghaiTech University established the photocatalytic aerobic
ring expansion of the
cycloheptanone 21 to the
macrolactone 22
(Angew. Chem. Int. Ed. 2021, 60, 5370.
DOI: 10.1002/anie.202012720).
Xiao-Qiang Hu of the South-Central University of
Nationalities, Guodong Zhang of Yanzhou University and Yang Gao of the Guangdong
University of Technology prepared the macrolactone 25 by coupling the benzoic
acid 23 with the chloroalkyne 24
(Chem. Commun. 2021, 57, 1113.
DOI: 10.1039/D0CC07573F).
Jiahai Zhou of the Shanghai Institute of Organic Chemistry and K. N. Houk and Yi Tang of UCLA
optimized an enzyme for the cyclization of the hydroxy acid 26 to the medium-ring lactone 27
(J. Am. Chem. Soc. 2021, 143, 80.
DOI: 10.1021/jacs.0c11226).
Wei Zhang of the University of Colorado showed that the Mo-catalyzed
ring-closing metathesis of the diyne
28 to the bis lactone 29 proceeded efficiently even in the presence of air
(Nature Commun. 2021, 12, 1136.
DOI: 10.1038/s41467-021-21364-4).
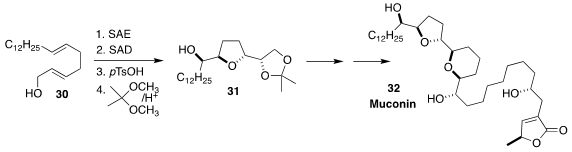
Muconin (32), isolated from the American pawpaw Asimina triloba, showed potent
cytotoxicity against human pancreatic and breast tumor cells. Hidefumi Makabe of
Shinshu University constructed the initial tetrahydrofuran segment 31 of
32 from
the simple precursor 30 by sequential
Sharpless asymmetric epoxidation and
Sharpless asymmetric dihydroxylation
(J. Org. Chem. 2021, 86, 4859.
DOI: 10.1021/acs.joc.0c02831).
For an early report of this strategy, see J. Org. Chem. 1994, 59,
3442 (DOI: 10.1021/jo00091a038).