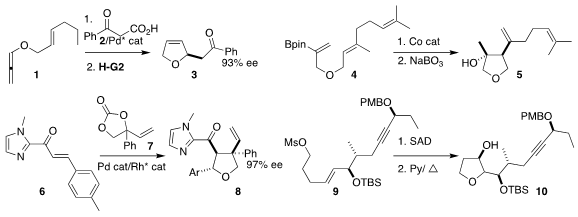
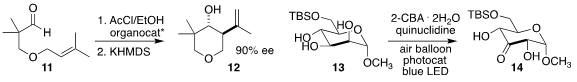Young Ho Rhee of the Pohang University of Science and Technology assembled
the dihydrofuran
3 by Pd-mediated coupling of the allene 1 with the keto acid 2,
followed by ring-closing metathesis
(Angew. 3-Hydroxy-1-methylazetidine Order Chem. Int. Ed. 2021, 60, 22166.
DOI: 10.1002/anie.202107990).
Jack R. Norton of Columbia University used a Co catalyst followed by oxidation
to convert the alkenyl borate ester 4 to the
tetrahydrofuran 5
(Angew. Chem. Int. Ed. 2021, 60, 22678.
DOI: 10.1002/anie.202107665).
Yu Du and Weiping Su of the Fujian Institute of Research on the Structure of Matter
used a combination of Pd and Rh catalysis to construct the tetrahydrofuran 8
by the coupling of the enone 6 with the carbonate 7
(Chem. 1349151-98-9 custom synthesis Eur. J. 2021, 27, 12742.
DOI: 10.1002/chem.202102024).
Wei-Min Dai of the Hong Kong University of Science and Technology showed that the diol from
Sharpless asymmetric dihydroxylation
of the mesylate 9 selectively cyclized to the tetrahydrofuran 10
(Org. Chem. PMID:28739548 Front. 2021, 8, 6491.
DOI: 10.1039/D1QO01049B).
Eric N. Jacobsen of Harvard University devised a thiourea catalyst that promoted enantioselective
Prins cyclization
of the aldehyde 11 to afford a mixture of the
tetrahydropyran
12 and the corresponding tertiary chloride,
the latter being converted to 12 in a subsequent elimination step
(J. Am. Chem. Soc. 2021, 143, 20077.
DOI: 10.1021/jacs.1c10890).
Mark S. Taylor of the University of Toronto showed that the
photo-oxidation of the protected sugar 13 to the 3-ketone 14 proceeded via
initial oxidation to the 2-ketone, followed by rearrangement
(Chem. Commun. 2021, 57, 12135.
DOI: 10.1039/D1CC05124E).
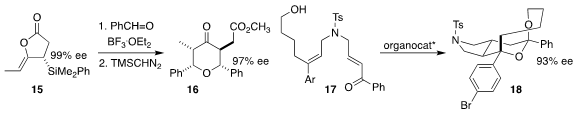
Kazuhiko Sakaguchi of Osaka City University prepared the
tetrahydropyranone 16 by combining the enantiomerically-pure allyl silane
15 with two equivalents of benzaldehyde
(J. Org. Chem. 2021, 86, 11884.
DOI: 10.1021/acs.joc.1c01284).
Wenjing Zhang of Zhengzhou University and Zhili Zuo and Liang-Liang Wang of the Kunming
Institute of Botany used an enantiomerically-pure phosphoric acid to cyclize the
dienyl alcohol 17 to the tricyclic spiroketal 18
(Nature Commun. 2021, 12, 7188.
DOI: 10.1038/s41467-021-27521-z).
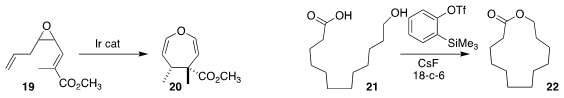
Ilan Marek of Technion showed that Ir-mediated alkene migration of the diene epoxide 19 led, via
Cope rearrangement, to the 4,5-dihydrooxepine 20 with high
diastereoselectivity
(Chem. Sci. 2021, 12, 9328.
DOI: 10.1039/D1SC02575A).
Jiarong Shi of Chongqing University and Yang Li of Jilin University used in situ-generated benzyne to
cyclize the hydroxy acid 21 to the
macrolactone 22
(Org. Lett. 2021, 23, 7274.
DOI: 10.1021/acs.orglett.1c02702).
In honor of the late Jiro Tsuji, it should be noted that he reported ring
closing metathesis to form a macrolactone more than forty years ago
(Tetrahedron Lett. 1980, 21, 2955.
DOI: 10.1016/0040-4039(80)88007-5).
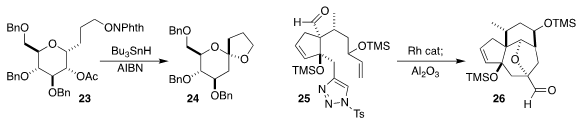
Inés Pérez-Martín and Ernesto Suárez of the Instituto de Productos Naturales
y Agrobiología del CSIC devised free radical conditions for cyclizing the
N-alkoxyphthalimide 23 to the spiroketal 24
(J. Org. Chem. 2021, 86, 14508.
DOI: 10.1021/acs.joc.1c01376).
Chuang-Chuang Li of the Southern University of Science and Technology assembled
the tetracyclic ether 26 by the Rh-mediated [3+2] cycloaddition of the
N-sulfonyltriazole 25
(Org. Lett. 2021, 23, 7771.
DOI: 10.1021/acs.orglett.1c02784).
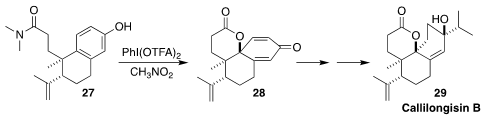
Callilongisin B (29), isolated from the Chinese flowering shrub Callicarpa
longissima, showed significant cytotoxicity and anti-inflammatory activity. In
the course of a synthesis of 29, Hisanaka Ito of the Tokyo University of
Pharmacy and Life Sciences achieved high diastereoselectivity in the oxidative
cyclization of the amide 27 to the lactone 28
(Org. Lett. 2021, 23, 6916.
DOI: 10.1021/acs.orglett.1c02473).