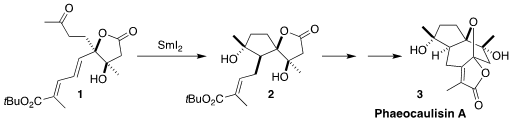
Phaeocaulisin A (3), isolated from Curcuma phaeocaulis, a Chinese
flowering plant in the ginger family, has been demonstrated to have promising
anti-neoplastic activity. PMID:24423657 876379-79-2 manufacturer David J. Procter of the University of Manchester
devised a synthetic route to 3 based on the SmI2-mediated reductive cyclization
of the dienyl ketone 1 to the
cyclopentanol
2
(J. Am. Chem. 1H-Imidazole-2-carbaldehyde web Soc. 2022, 144, 7457.
DOI: 10.1021/jacs.2c02188).
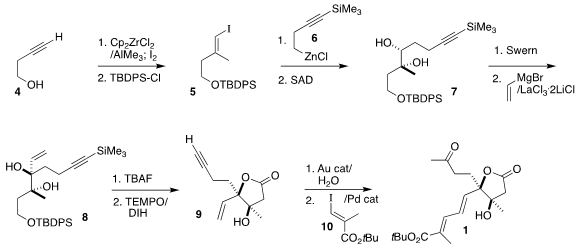
The assembly of 1 began with the preparation of the silyl ether 5 by Negishi
iodomethylation of the butynol 4 followed by protection. Coupling with 6 gave
the enyne, that was
dihydroxylated under the enantioselective Sharpless
conditions, leading to the diol 7. Oxidation followed by the addition of vinyl
magnesium bromide delivered the key diol 8 with high diastereocontrol.
Desilylation led to the crystalline triol, that was oxidized to the lactone
9 with
TEMPO and
1,3-diiodo-5,5-dimethylhydantoin. Hydration of the alkyne to the methyl ketone followed by Heck coupling with the iodide
10 completed the
construction of the diene 1.
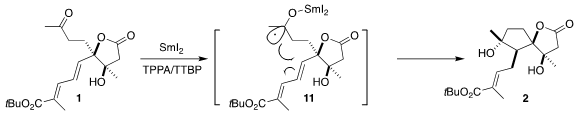
Reduction of 1 with
SmI2 could proceed via initial complexation with any of
the three carbonyls. Initially, the methyl ester was explored, but this gave
little of the desired cyclization. Fortunately, the t-butyl ester was successful,
suggesting that the bulky ester is blocking complexation of the SmI2 to that
carbonyl.
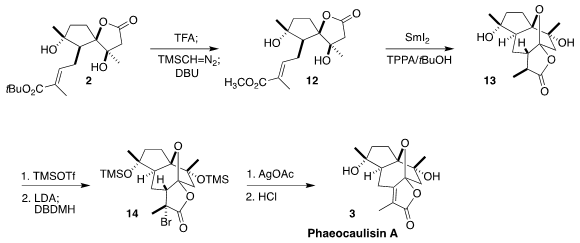
The last rings of 3 were also to be assembled by SmI2 reduction. The bulky
t-butyl ester would not participate, so 2 was converted to the methyl ester
12.
Cyclization then proceeded smoothly to give the lactone, largely as diasteromer
13. The bottom face of the derived enolate was more open, so bromination with
1,3-dibromo-5,5-dimethylhydantoin proceeded to give the bromide
14. Ionization
with stoichiometric silver then led to elimination to give, after deprotection,
phaeocaulisin A (3).
The synthesis of phaeocaulisin A (3) outlined here is a tribute to the many
years the Procter group has given to developing the chemistry of SmI2 as a reagent for organic synthesis.