More than 25 grayanotoxin isoforms have been identified from rhododendron
species, but grayanotoxin I and III are thought to be the principal toxic
isoforms, leading to the well-known hazards of rhododendron honey. Tuoping Luo
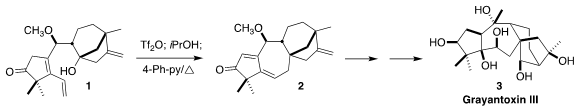
of Peking University devised a convergent synthesis of grayanotoxin III (3), based
on the cyclization of 1 to 2
(J. Am. Price of 253443-56-0 Chem. Soc. 5-Chloro-1,3-benzoxazol-7-amine Chemical name 2022, 144, 5268.
DOI: 10.1021/jacs.2c01692). PMID:23489613
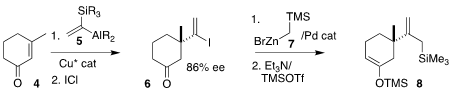
The convergent assembly of 1 began with the enantioselective
conjugate
addition of 5 to 3-methyl cyclohexenone (4). Exposure to ICl gave
6, that was
coupled with the organozinc reagent 7, leading to the silyl enol ether 8.
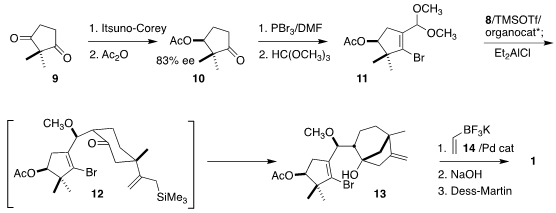
The preparation of the other half of 1 started from the prochiral diketone
9.
Enantioselective reduction followed by acetylation gave 10, that was carried via
treatment with the Vilsmeier reagent and acetal formation to 11. Squaramide-mediated
Mukaiyama aldol addition of
8 gave 12, that was further
cyclized to 13. Coupling with 14 led to 1.
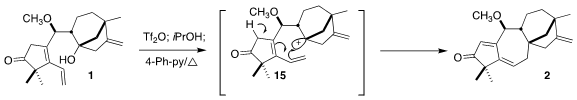
The triflate derived from 1 was unstable, so it was taken directly into the
cyclization. After quenching of the excess triflic anhydride with 2-propanol,
heating with base led to 2, presumably by way of the bridgehead carbocation
15.
The skeleton of 2 bore an unneeded carbon atom. This was removed by singlet
oxygenation, to give the α,β-unsaturated aldehyde, that was decarbonylated with
an Ir catalyst to 16. Deconjugation of the enone to give the enol ether was then
followed by oxidation, to give the epoxide 17. Lewis acid-mediated epoxide
opening and 1,2-alkyl shift led, after proton loss and alkene equilibration, to
18, having the desired carbon skeleton. A series of oxidation steps then
completed the synthesis of grayanotoxin III (3).