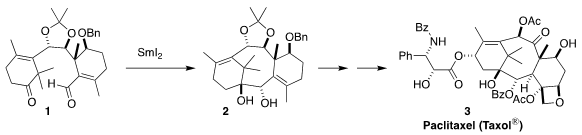
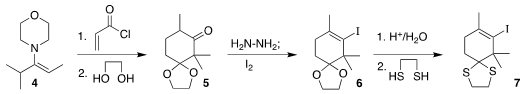Paclitaxel (Taxol®) (3) is a critical tool for cancer chemotherapy. PMID:27108903
Chuang-Chuang Li of the Southern University of Science and Technology developed a convergent
approach to 3, based on the reductive cyclization of 1 to 2
(J. Am. Chem. Buy1H-Imidazole-2-carbaldehyde Soc. 2021, 143, 17862.
DOI: 10.1021/jacs.1c09637).
The construction of 1 goes back to the work of P. W. Hickmott of the
University of Salford
(J. Buy1018295-42-5 Chem. Soc. (C) 1968, 2599.
DOI: 10.1039/J39680002599).
Acylation of the
morpholine enamine 4 with acryloyl chloride followed by hydrolysis and
ketalization
led to 5. Based on other literature precedent, exposure of the derived
hydrazone to I2 led to 6, that was hydrolyzed and
protected as the
thioketal 7.
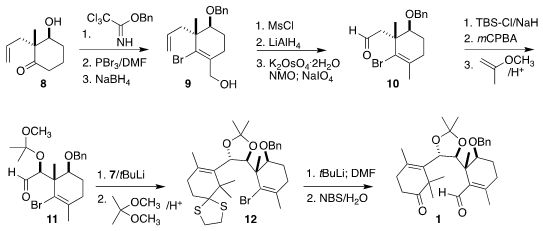
The preparation of the other half of 1 began with the commercial
ketone 8, prepared in enantiomerically-pure form by reduction of the
1,3-diketone.
Benzylation followed by
Vilsmeier reaction
and reduction led to the alcohol 9. Reduction of the derived mesylate
followed by
oxidative cleavage delivered the aldehyde 10.
Rubottom oxidation of
the silyl enol ether proceeded with high diastereoselectivity, to give, after
protection, the bromide 12, that was formylated and deprotected to give
1.
Despite the literature precedent for the reductive cyclization of similar but
less substituted keto aldehydes, the conversion of 1 to 2 was
particularly challenging. Eventually, it was found that adding Sm metal to the
medium led to efficient conversion. The diol 2 was not stable, so it was
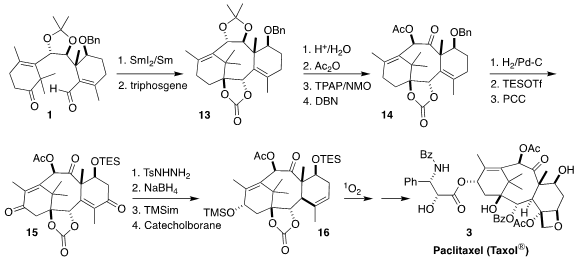
protected, leading to the crystalline 13.
Hydrolysis, selective acetylation and oxidation of 13 led to the
monoketone, that was epimerized to the more stable 14. Debenzylation,
silylation and oxidation then gave the triketone 15. Selective
tosylhydrazone formation,
reduction and protection set the stage for Kabalka reduction, leading to the
requisite trans-fused alkene 16. Singlet oxygenation followed by a
modification of the literature endgame then completed the synthesis of
paclitaxel 3.
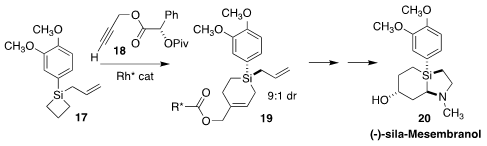
Silicon-based lifeforms? Maybe not, but silicon-based pharmaceuticals may not
be far off. Ruotian Jiang and Zhenlei Song of Sichuan University showed that
sila-mesembranol (20), prepared by combining the prochiral silane 17
with the propargyl ester 18 to give 19, retained the
anti-anxiolytic activity of the Sceletium alkaloid
(Org. Chem. Front. 2021, 8, 5941.
DOI: 10.1039/D1QO00682G).