Helionine (3) was isolated from the Chinese lily Fritillaria ussuriensis, used
in traditional medicine. Viresh H. Buy1,3,5-Tri(pyridin-4-yl)benzene Rawal of the University of Chicago devised a convergent approach
to 3, based on the cyclization of 1 to
assemble the central benzene ring of 2
(J. PMID:24101108 Am. Chem. Soc. 2021, 143, 16394.
DOI: 10.1021/jacs.1c08756).
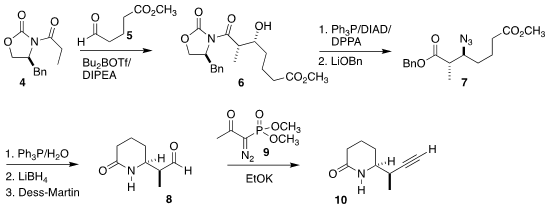
The construction of the crystalline lactam 10 began with the Evans aldol
addition of the acyl oxazolidinone 4 to the aldehyde 5 to give 6. 1416990-09-4 web This was carried on
to the azide
7, that was cyclized, reduced and oxidized to the
aldehyde 8. Conversion of the aldehyde to the alkyne with the
Ohira-Bestmann
reagent (9) then completed the preparation of 10.
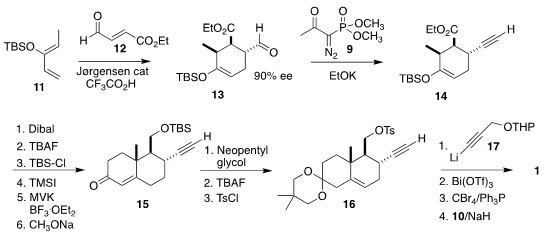
The assembly of the bicyclic alkyne 1 began with the
Diels-Alder
cycloaddition of 11 with 12 to give 13. Again, application of the Ohira-Bestmann
reagent led to the enol ether 14. Hydrolysis followed by
re-silylation gave the
alternative enol ether, that was condensed with methyl vinyl ketone to give a
diketone that was cyclized to the enone 15. Protection of the enone then led to
the tosylate 16, that was used to alkylate the linchpin reagent 17. Hydrolysis
and exposure to Ph3P/CBr4 gave a bromide that was coupled with
10 to complete
the preparation of 1.
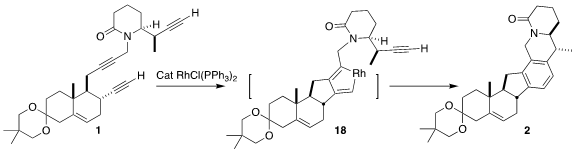
The Rh-catalyzed cyclization of 1 probably proceeded via initial coupling of
the two proximal alkynes to give an intermediate such as 18. That put the third
alkyne within reach, leading to 2.
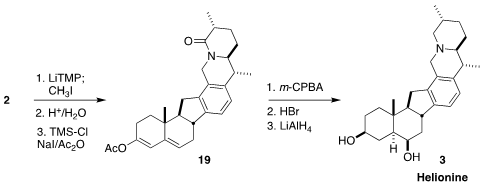
The lactam 2 was methylated, and the ketone was deprotected, leading to the
conjugated enone, that was converted to the enol acetate 19.
Epoxidation,
hydrolysis and β-elimination led to an allylic alcohol, that on acid-mediated
enolization gave the corresponding 1,4-diketone. Reduction completed the
synthesis of helionine (3).