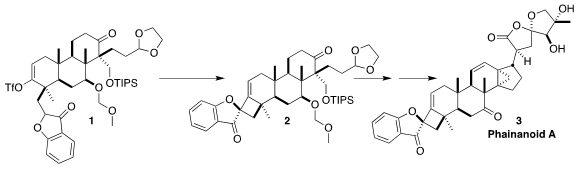
Phainanoid A (3), isolated from the shrub Phyllanthus hainanensis
Merr., found only on Hainan Island, showed both promising toxicity against
cancer cell lines, and immunosuppressive activity. Guangbin Dong of the
University of Chicago faced many challenges in assembling 3, including the
cyclization of the ketone 1 to the
cyclobutane 2
(J. PMID:23557924 4-(Difluoromethyl)-3-fluorobenzoic acid structure Am. Chem. 2151915-22-7 site Soc. 2021, 143, 19311.
DOI: 10.1021/jacs.1c11117).
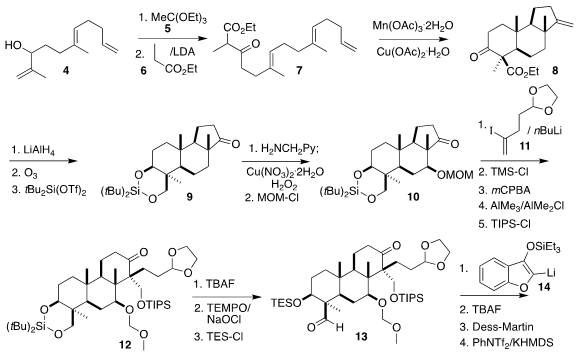
The construction of the triflate 1 began with the triene alcohol 4.
Claisen
rearrangement with 5 followed by the addition of the ester enolate derived from
6 led to the β-keto ester 7. Oxidative
cyclization led to the tricyclic ketone 8 with the expected high
diastereocontrol. Remote
hydroxylation of the derived ketone
9 led to 10, that
was coupled with the iodoalkene 11. Protection followed by
epoxidation and
semi-pinacol
rearrangement then established the correct diastereomer of the ketone
12. Deprotection of the diol followed by selective oxidation and protection gave
the aldehyde
13. Coupling with the benzofuran 14 then led to 15.
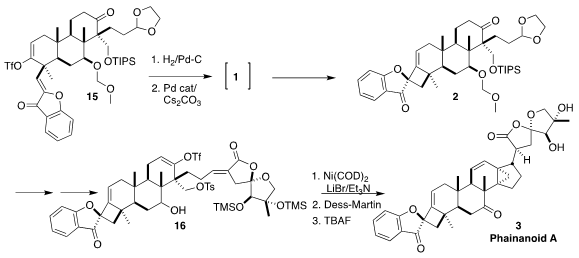
Reduction of 15 led to 1, that was directly carried on to 2. The Pd-catalyzed
cyclization proceeded with high diastereoselectivity, with the bulky aryl ketone
on the more open face of the cyclobutane.
The last stage of the assembly of 3 was the Ni-mediated cyclization of
16.
This apparently proceeded by initial enagement of the vinyl triflate with the
exo alkylidene lactone. Subsequent
Heck-type cyclization then established the
cyclopropane.
Both the ketone 2 and the phosphonate used to carry it on to 16 were racemic,
so 16 was a mixture of racemic diastereomers, the correct one of which was
converted to racemic 3. If instead a single enantiomer of the phosphonate had
been used, the yield of phainanoid A (3) would have been the same, but it would
have been enantiomerically pure.
We note the passing of David A. Evans of Harvard University. In addition to
their many contributions to organic synthesis, we all owe a debt of gratitude to
the Evans group and Stewart Rubenstein for the development of ChemDraw.