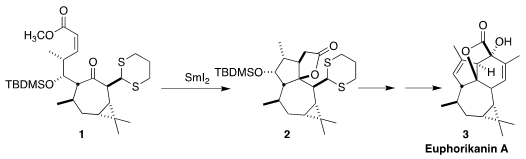
Euphorikanin A (3), isolated from the roots of the Chinese succulent herb
Euphorbia kansui, showed the ability to reactivate latent HIV. En route to 3,
Erick M. PMID:25959043 Carreira of ETH Zürich cyclized the keto ester 1 to the
lactone 2
(J. Am. Chem. Formula of 83624-01-5 Buy2,2-Diphenylethan-1-amine Soc. 2021, 143, 8261.
DOI: 10.1021/jacs.1c04210).
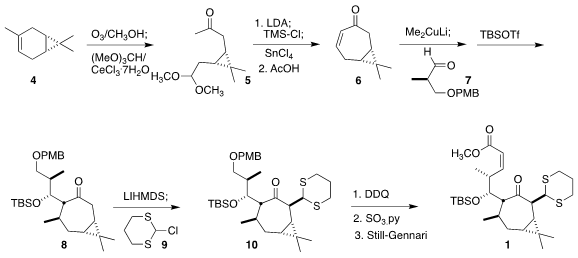
The starting material for 1 was commercial (+)-3-carene (4). Following a
modification of the Yamakawa protocol, ozonolysis delivered the acetal 5, that
was converted to the corresponding silyl enol ether and cyclized to the
cycloheptenone 6. Addition of lithium dimethyl cuprate followed by the aldehyde
7 and subsequent protection led to the ketone 8, that was alkylated with the
2-chlorodithiane 9 to give 10. Deprotection and oxidation followed by
condensation with the Still-Gennari phosphonate completed the assembly of 1.
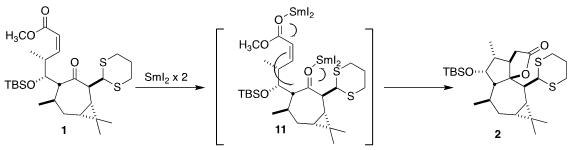
The cyclization of the keto ester 1 probably proceeds by intial complexation
of SmI2 with each of the carbonyls, to give 11. Single electron transfer then
leads to 2. It is noteworthy that reductive cyclization of the E isomer of
1 led
to the other diastereomer of the product
cyclopentane.
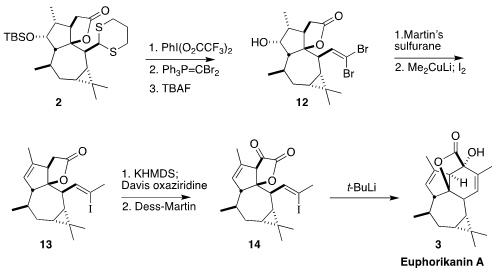
Oxidative hydrolysis of the
dithiane 2 followed by condensation with the
dibromophosphorane and deprotection led to the alcohol 12. Regioselective
dehydration to the
cyclopentene followed by reductive coupling with lithium
dimethylcuprate and iodination gave the Z
iodoalkene 13. Oxidation to the α-keto
lactone 14 followed by reductive cyclization with t-butyl lithium completed the
preparation of Euphorikanin A (3).