Mitrephorone A (Carreira)
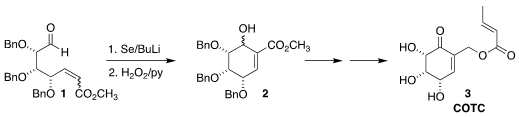
The crotonate ester COTC (3), an inhibitor of alkaline
phosphodiesterase isolated from a soil Streptomyces, had cytotoxic and
cancerostatic activity. Szymon Buda of Jagiellonian University prepared the
carbocyclic core 2 of 3 by cyclizing the ribose-derived aldehyde
1 with selenide (Tetrahedron 2020, 76, 131397.
DOI: 10.1016/j.tet.2020.131397).
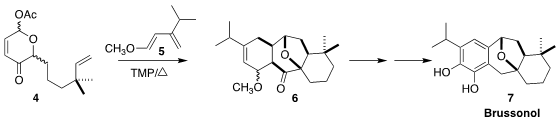
The icetexane diterpene brussonol (7), isolated from Russian sage,
Perovskia atriplicifolia, is moderately cytotoxic toward P388 murine
leukemia cells. PMID:24507727 1H-Imidazole-2-carbaldehyde In stock Yehua Jin of Launch Pharma Technologies and Fayang G. Qiu the
Guangzhou Institutes of Biomedicine and Health assembled the tetracyclic
skeleton 6 by the addition of the oxidopyrylium derived from 4 to
the diene 5
(Org. tert-Butyl 3-bromopropanoate Chemical name Lett. 2020, 22, 7415.
DOI: 10.1021/acs.orglett.0c02309).
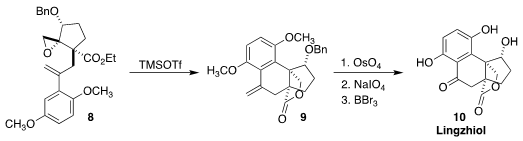
Lingzhiol (10), isolated from the medicinal reishi fungus Ganoderma
lingzhi, ameliorated adriamycin-induced nephropathy in mice. Sanzhong Luo of
Tsinghua University and Hong-Bo Qin of the Kungmin Institute of Botany
constructed the carbon skeleton of 10 by the diastereoselective
cyclization of 8 to 9
(Chem. Commun. 2020, 56, 10066.
DOI: 10.1039/D0CC04064A).
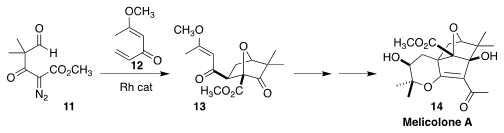
Melicolone A (14), isolated from the leaves of the dioecious medicinal
shrub Melicope ptelefolia of Southeast Asia, showed a protective activity
against high glucose-induced oxidative stress in human vein endothelial cells.
En route to 14, Stephen F. Martin of the University of Texas showed that the
carbonyl ylide derived from 11 added to the dienone 12 to give
13 with high regio- and diastereoselectivity
(Org. Lett. 2020, 22, 9071.
DOI: 10.1021/acs.orglett.0c03454).
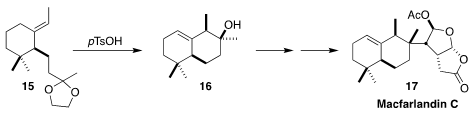
Macfarlandin C (17), isolated from the dorid nudibranch Chromodoris
macfarlandi, induced irreversible fragmentation of the Golgi apparatus.
Larry E. Overman of the University of California, Irvine established the octalin
core 16 by the acid-catalyzed cyclization of 15, prepared in high ee
(J. Org. Chem. 2020, 85, 15532.
DOI: 10.1021/acs.joc.0c02273).
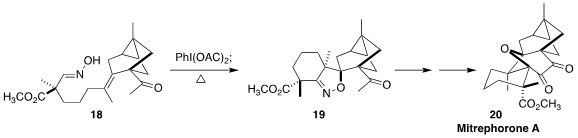
The total synthesis of mitrephorone A (21) has previously been
addressed in these Highlights
(![]() 2019, August 19;
2019, August 19;
![]() 2020, August 24).
2020, August 24).
Erick M. Carreira of ETH Zürich established an improved approach, based on the diastereoselective
dipolar
cycloaddition of the nitrile oxide derived from 18, to give 19
(J. Am. Chem. Soc. 2020, 142, 17802.
DOI: 10.1021/jacs.0c09520).