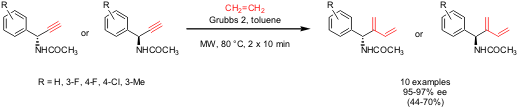
Maurizio Botta and his group from the University of Siena, Italy have employed microwave mediated ethylene-alkyne cross-metathesis using Grubbs 2 catalyst for the synthesis of enantioenriched 2-substituted butadiens (Tetrahedron: Asymmetry 2005, 16, 2893. PMID:23789847 1073371-77-3 manufacturer DOI: 10.1016/j.tetasy.2005.08.002). Importantly, microwave heating was essential for the fast reaction of enantiomerically enriched alkynes with ethylene (closed vessel saturated with ethylene) under retention of configuration at the propargylic/allylic position. 7-Bromo-1H-pyrazolo[3,4-c]pyridine web At atmospheric pressure, no reaction was observed.
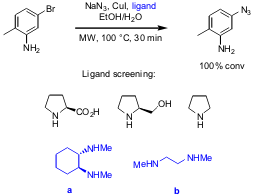
Synthesis of Aryl Azides under Mild Conditions
In a recent publication, Xifu Liang and co-workers from LEO Pharma, Denmark, have reported on optimization studies for the synthesis of aryl azides from aryl halides using copper(I) catalysis (Synlett 2005, 2209.DOI: 10.1055/s-2005-872248). In a first screening several ligands have been tested, resulting in the selection of 1,2-diamino ligands a and b. In further optimizations, different solvents and catalyst/ligand ratios have been studied, reaching full conversion by using EtOH/H2O as solvent mixture, 10 mol % of CuI, 15 mol % of ligand b and irradiation at 100 °C for 30 minutes.
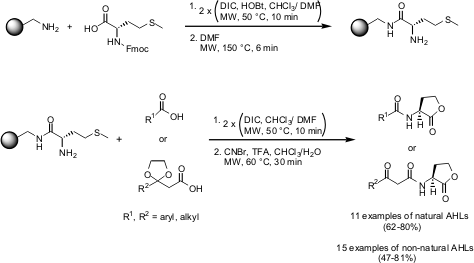
Solid-Phase Synthesis of Natural and Non-Natural AHLs
Helen E. Blackwell and co-workers from the University of Wisconsin-Madison have described the synthesis of N-acyl L-homoserine lactones (AHLs) via a microwave-assisted solid-phase route (J. Am. Chem. Soc. 2005, 127, 12762.DOI: 10.1021/ja0530321). Both natural and non-natural AHLs could be obtained in high purities and short reaction times. Among the two libraries, a set of non-native AHLs has been found to have high inhibitory affinity for bacterial quorum sensing.
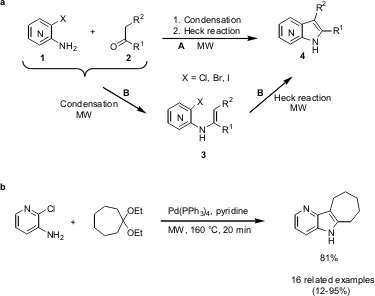
Synthesis of 4-, 5-, 6- and 7-Azaindoles
Nicolas Lachance and co-workers form the Merck Frosst Centre for Therapeutic Research in Quebec (Canada) have shown that imines/enamines 3 can be converted via a microwave-assisted intramolecular Heck reaction to the corresponding azaindoles 4 in good yields (Synthesis 2005, 2571. DOI: 10.1055/s-2005-872100). Several synthesis routes have been investigated, including the one-step reaction (route A) of aminopyridines 1 with ketones 2 or ketals respectively as well as the one-pot two-step reaction (route B) leading to azaindoles 4. In this way a small library of 17 examples was synthesized employing chloro-, bromo- and iodoaminopyridines with toleration of sensitive groups like ketones and esters.