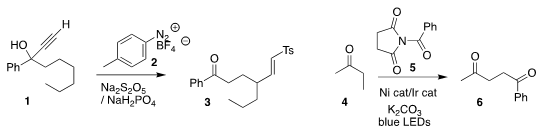
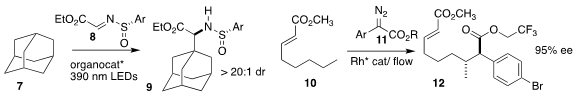
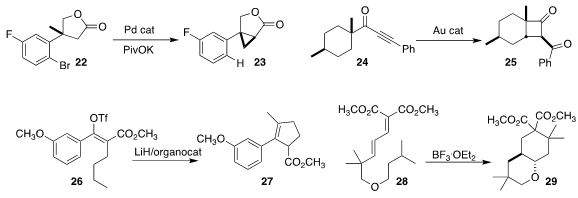
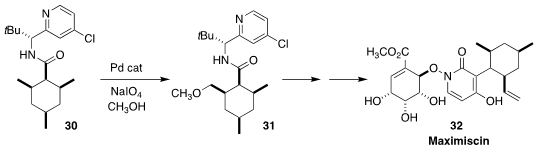
Jie Wu of Taizhou University prepared the
vinyl sulfone 3 by coupling the
tertiary propargylic alcohol 1 with the
diazonium salt 2 in the presence of
sodium metabisulfite
(Adv. Synth. Catal. 2020, 362, 4744.
DOI: 10.1002/adsc.202000778).
Mu-Hyun Baik and Soon
Hyeok Hong of KAIST observed high selectivity for the
1,4-diketone 6 from the
acylation of the ketone 4 wth the succinylamide 5
(Angew. Chem. Int. 2-Bromo-5-(difluoromethyl)pyrazine structure Ed. 2020, 59, 16933.
DOI: 10.1002/anie.202004441).
David B. C. Martin of the University of Iowa achieved high diastereoselectivity in
the coupling of 7 with 8, leading to the sulfinylamide 9
(Chem. Commun. 82979-45-1 web 2020, 56, 9699.
DOI: 10.1039/D0CC02804E).
Huw M. L. Davies of Emory University and Christopher W. Jones of the Georgia Institute of Technology
designed a Rh catalyst that worked well in flow for the diastereoselective and enantioselective
coupling of the diazo ester 11 with just one of the 16 C-H bonds of 10
(Angew. Chem. Int. PMID:23724934 Ed. 2020, 59, 19525.
DOI: 10.1002/anie.202005381).
Sukbok Chang, also of KAIST, cyclized the 1,4-2-dioxol-5-one to the
γ-lactam 14
(J. Am. Chem. Soc. 2020, 142, 8880.
DOI: 10.1021/jacs.0c02079).
Bing-Feng Shi of Zhejiang University assembled the γ-lactam
17 by coupling the amide 15 with the alkenyl iodide 16
(Angew. Chem. Int. Ed. 2020, 59, 14060.
DOI: 10.1002/anie.202004504).
Donovon Adpressa and Charles S. Yeung of Merck and Richmond Sarpong of
the University of California, Berkeley cyclized the sulfonamide 18 to the
pyrrolidine 19
(Org. Lett. 2020, 22, 6578.
DOI: 10.1021/acs.orglett.0c02345).
Chi-Ming Che of the University of Hong Kong used visible light to promote the Fe-catalyzed
cyclization of the α-azido ketone 20 to the pyrrolidine 21
(Chem. Sci. 2020, 11, 4680.
DOI: 10.1039/D0SC00784F).
Olivier Baudoin of the University of Basel used the proximal bromo arene to
oxidize the γ-lactone 22 to the
cyclopropane 23
(J. Am. Chem. Soc. 2020, 142, 15355.
DOI: 10.1021/jacs.0c05887).
Yuxue Li of the Shanghai Institute of Organic Chemistry and Liming Zhang
of the University of California, Santa Barbara employed a gold catalyst to
cyclize the alkynyl ketone 24 to the α-benzoyl
cyclobutanone 25
(Angew. Chem. Int. Ed. 2020, 59, 17398.
DOI: 10.1002/anie.202003698).
Hosea M. Nelson of UCLA used a urea catalyst to
cyclize the alkenyl triflate 26 to the
cyclopentene 27
(Org. Lett. 2020, 22, 7775.
DOI: 10.1021/acs.orglett.0c01745).
Keiji Mori of the Tokyo University of Agriculture and Technology showed
that a Lewis acid was sufficient to cyclize the dienyl diester 28 to the
cyclohexane 29
(Org. Lett. 2020, 22, 5801.
DOI: 10.1021/acs.orglett.0c01867).
Maximiscin (32), an antibiotic derived from an Alaskan soil sample, showed
significant anti-cancer activity. En route to 32, Phil S. Baran of Scripps/La Jolla
achieved high diastereoselectivity in the oxidation of 30 to 31
(J. Am. Chem. Soc. 2020, 142, 8608.
DOI: 10.1021/jacs.0c03202).