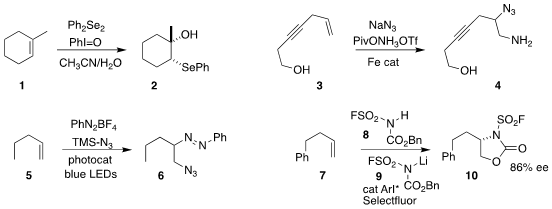
Gong-Qing Liu and Yong Ling of Nantong University prepared the selenyl alcohol 2
by the oxidative addition of diphenyl diselenide to the alkene 1
(J. Org. PMID:24580853 Chem. 2021, 86, 5292.
DOI: 10.1021/acs.joc.1c00257).
Bill Morandi of ETH Zürich effected the selective
azido amination of the alkene 3, leading to 4
(J. Am. Price of (+)-Sparteine Chem. Soc. 2020, 142, 21548.
DOI: 10.1021/jacs.0c11025).
Wanmei Liu and Pengfei Zhang of Hangzhou Normal University and Qing Zhu
of the Zhejiang University of Technology combined the alkene 5 with TMS azide
and an aryl diazonium salt
to give the dialkyl diazene 6
(Org. Lett. 2021, 23, 1204.
DOI: 10.1021/acs.orglett.0c04148).
Takuya Hashimoto of Chiba University assembled the
cyclic urethane
10 by the addition of the sulfonamide 8 to the alkene 7
(J. Am. Chem. Soc. 2021, 143, 1745.
DOI: 10.1021/jacs.0c11440).
Frank Glorius of the Westfälische Wilhelms-Universität Münster described
a parallel investigation
(Nature Catal. 2021, 4, 54.
DOI: 10.1038/s41929-020-00553-2). 22112-84-1 Order
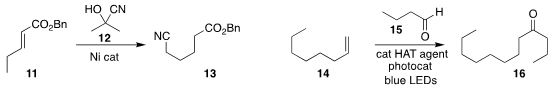
Gui-Juan Cheng of the Chinese University of Hong Kong and Xianjie Fang of
Shanghai Jiao Tong University developed a Ni catalyst for the selective terminal
migratory hydrocyanation of an internal alkene 11 with acetone cyanohydrin
12, leading to the cyano ester 13
(Org. Lett. 2021, 23, 486,
DOI: 10.1021/acs.orglett.0c04007;
Angew. Chem. Int. Ed. 2021, 60, 1883,
DOI: 10.1002/anie.202011231).
Joyram Guin of the Indian Association for the Cultivation of
Science devised the assembly of the ketone 16 by the visible light-promoted
coupling of the alkene
14 with the aldehyde 15
(Chem. Eur. J. 2021, 27, 4412.
DOI: 10.1002/chem.202004946).
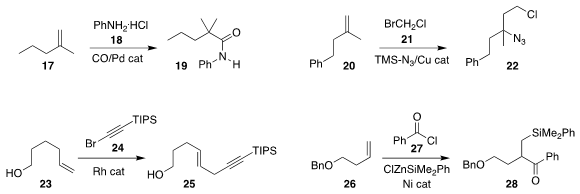
Zheng-Hui Guan of Northwest University developed the Pd-catalysed Markovnikov carbonylative
coupling of the alkene 17 with aniline 18, leading to the amide 19
(J. Am. Chem. Soc. 2021, 143, 7298.
DOI: 10.1021/jacs.1c03454).
Xi-Sheng Wang of the University of Science
and Technology of China used a Cu catalyst to mediate the assembly of the chloro
azide 22 by the combination of the alkene 20, bromochloromethane
21 and trimethylsilyl azide
(Chem. Commun. 2021, 57, 5666.
DOI: 10.1039/D1CC01751A).
Antonio M. Echavarren of ICIQ coupled the alkene 23 with the bromoalkyne 24
to give the enyne 25
(Angew. Chem. Int. Ed. 2021, 60, 5693.
DOI: 10.1002/anie.202014877).
Professor Glorius reported related results
(Angew. Chem. Int. Ed. 2021, 60, 5688.
DOI: 10.1002/anie.202015249).
M. Kevin Brown of Indiana University assembled
the β-silyl ketone 28 by the addition of ClZnSiMe2Ph and benzoyl chloride
27 to the alkene 26
(ACS Catal. 2021, 11, 1858.
DOI: 10.1021/acscatal.0c05449).
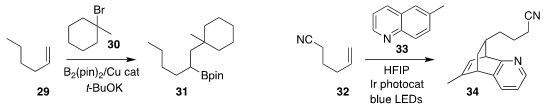
Hajime Ito of Hokkaido University prepared the
boronate
31 by the Cu-catalyzed
addition of the tertiary bromide 30 to the alkene 29
(J. Am. Chem. Soc. 2021,
143, 5260.
DOI: 10.1021/jacs.1c02050).
Professor Brown, Professor Glorius and Kendall N. Houk of UCLA
uncovered the photochemically-promoted construction of the tricyclic
pyridine 34 by the cycloaddition of the
quinoline
33 to the alkene 32
(Science 2021, 371, 1338.
DOI: 10.1126/science.abg0720).
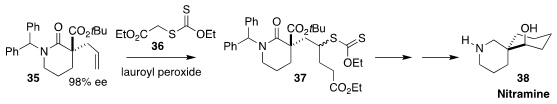
Many of the Nitraria alkaloids, exemplified by nitramine 38, contain the
2-azaspiro[5,5]undecane ring structure. In the course of a synthesis of 38, Hyeung-geung Park of Seoul National University coupled the readily-prepared
alkene 35 with the Zard reagent 36, leading to 37
(J. Org. Chem. 2021, 86, 4375.
DOI: 10.1021/acs.joc.0c02573).