Walter Leitner of the Max Planck Institute for Chemical Energy Conversion and RWTH Aachen University
(Angew. Chem. Int. Ed. 2020, 59, 215.
DOI: 10.1002/anie.201909035)
and Rhett Kempe of the University of Bayreuth
(Angew. Chem. Grubbs 1st Purity Int. Ed. 2020, 59, 1485.
DOI: 10.1002/anie.201912055)
independently developed protocols for methylation
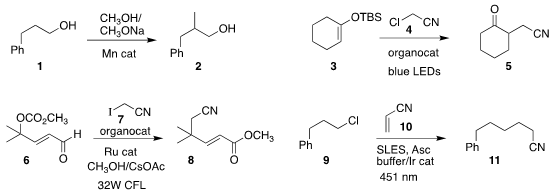
β to an alcohol, converting 1 to the branched product 2. Paolo Melchiorre of ICIQ assembled the
keto nitrile 5 by coupling the silyl enol ether 3 with 4
(Angew. Chem. Int. 5-Chloropyrimidin-2(1H)-one web Ed. PMID:23937941 2020, 59, 9485.
DOI: 10.1002/anie.201915814).
Zhong-Hua Gao and Song Ye of the Institute of Chemistry of the
Chinese Academy of Sciences used an NHC catalyst to prepare the cyano ester 8 by
coupling the carbonate 6 with 7
(Angew. Chem. Int. Ed. 2019, 58, 18124.
DOI: 10.1002/anie.201909017).
Werner Kunz and Burkhard König of the University of Regensburg devised conditions for
the conjugate addition of the chloride 9 to acrylonitrile 10, leading to 11
(Nature Catal. 2020, 3, 40.
DOI: 10.1038/s41929-019-0369-5).
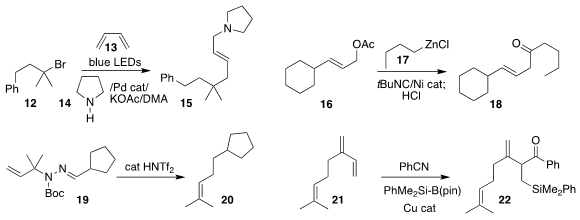
Frank Glorius of the Westfälische Wilhelms-Universität Münster assembled 15 by adding the bromide
12 to butadiene 13 in the presence of pyrrolidine (14)
(J. Am. Chem. Soc. 2020, 142, 10173.
DOI: 10.1021/jacs.0c03239).
Jingping Qu and Yifeng Chen of the East China
University of Science and Technology, using t-butyl isocyanide as a linchpin, prepared
the ketone 18 by coupling the allylic acetate 16 with the organozinc 17
(Nature Commun. 2020, 11, 392.
DOI: 10.1038/s41467-020-14320-1).
Franz Bracher of the Ludwig-Maxmilians University, Munich devised the traceless
elimination of the hydrazone 19 to give the alkene 20
(Eur. J. Org. Chem. 2020, 3680.
DOI: 10.1002/ejoc.202000382).
Tetsuaki Fujihara of Kyoto University converted the triene 21 to the silyl ketone 22
(Chem. Commun. 2020, 56, 4648.
DOI: 10.1039/D0CC01803A).
Zheng Li of Northwest Normal University used calcium carbide to convert the
tosylhydrazone derived from 23
to the alkyne 24
(Synlett 2019, 30, 1580,
DOI: 10.1055/s-0037-1610718;
Org. Chem. Front. 2020, 7, 702,
DOI: 10.1039/C9QO01400D).
Antonio M. Echavarren, also of ICIQ, effected the alkynylation of the carvone-derived
methoxime 25 with the bromoalkyne 26, leading to 27
(Angew. Chem. Int. Ed. 2020, 59, 10470.
DOI: 10.1002/anie.202002948).
Jieping Zhu of the Ecole Polytechnique Fédérale de Lausanne described a parallel investigation
(Nature Commun. 2020, 11, 403.
DOI: 10.1038/s41467-020-14292-2).
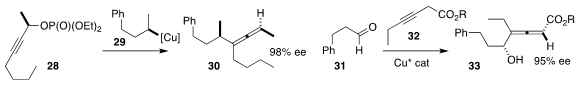
Paul Knochel, also of the Ludwig-Maxmilians University,
assembled the allene
30 by coupling the enantiomerically-enriched propargylic phosphate 28 with the
enantiodefined reagent 29
(Chem. Sci. 2020, 11, 5328.
DOI: 10.1039/C9SC05982B).
Liang Yin of the Shanghai Institute of Organic Chemistry used a Cu catalyst to mediate the preparation of
the allene 33 by the addition of the alkyne 32 to the aldehyde 31
(Angew. Chem. Int. Ed. 2020, 59, 1562.
DOI: 10.1002/anie.201912140).
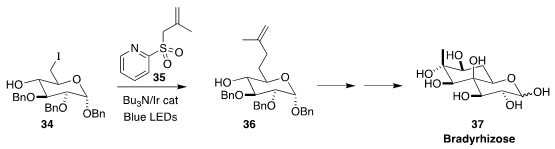
Bradyrhizose 37 is thought to be involved as a molecular signal in the
fixation of nitrogen in tropical Aeschynomenes species. In the course of a
synthesis of 37, David Crich of the University of Georgia showed that the
pyridyl sulfone 35 was an efficient radical acceptor for the conversion of
34 to 36
(Org. Lett. 2020, 22, 523.
DOI: 10.1021/acs.orglett.9b04279).