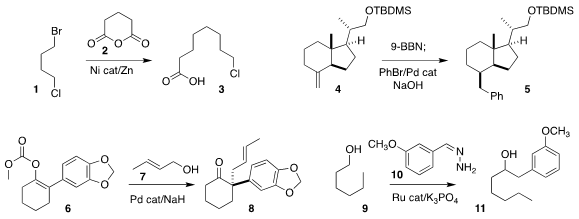
Patrick J. Walsh of the University of Pennsylvania and Jianyou Mao of Nanjing
Tech University assembled the carboxylic acid 3 by the decarboxylative coupling
of glutaric anhydride 2 with the bis-halide 1
(Nature Commun. 2020, 11, 5638.
DOI: 10.1038/s41467-020-19194-x).
Franz Bracher of Ludwig-Maximilians University observed high equatorial
selectivity in the conversion of 4 to 5 by sequential
hydroboration and
Suzuki-Miyaura cross-coupling
(Eur. J. Org. Chem. 2-Bromo-6-chloronicotinaldehyde Data Sheet 883-40-9 web 2020, 6270.
DOI: 10.1002/ejoc.202001080).
Alakesh Bisai of
the Indian Institute of Science Education and Research Bhopal prepared the
ketone 8 by the Pd-mediated coupling of the enol carbonate 6 with the allylic
alcohol 7
(Tetrahedron Lett. 2020, 61, 152129.
DOI: 10.1016/j.tetlet.2020.152129).
Chao-Jun Li of McGill University
devised a new method for carbon-carbon bond formation, the construction of 11 by
the Ru-catalyzed coupling of the alcohol
9 with the hydrazone 10
(Nature Commun. 2020, 11, 6022.
DOI: 10.1038/s41467-020-19857-9). PMID:23775868
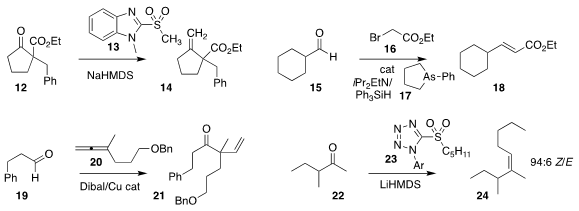
Kaori Ando of Gifu University devised an effective protocol for ketone
methylenation, demonstrating that
Julia-Kocienski olefination with the sulfone
13 converted even the very challenging substrate 12 to the alkene
14
(J. Org.
Chem. 2020, 85, 9936.
DOI: 10.1021/acs.joc.0c01227).
Hiroaki Imoto and Kensuke Naka of the Kyoto Institute of
Technology showed that the tetrahydroarsole 17 was an effective catalyst for the
arsa-Wittig reaction, mediating the
preparation of the unsaturated ester
18 by the coupling of the aldehyde 15 with the bromoester 16
(Chem. Eur. J. 2020, 26, 13400.
DOI: 10.1002/chem.202002792).
Yunmi Lee of Kwangwoon University employed excess aldehyde 19 in the reductive coupling with the allene
20, leading via subsequent
Oppenauer
oxidation to the ketone 21
(Org. Lett. 2020, 22, 5806.
DOI: 10.1021/acs.orglett.0c01876).
In another application of Julia-Kocienski olefination, Professor Ando showed that addition of the
sulfone 23 to the ketone 22 delivered predominantly the less-stable
Z alkene 24
(Org. Lett. 2020, 22, 6907.
DOI: 10.1021/acs.orglett.0c02440).
Amir H. Hoveyda of Boston College assembled a
detailed review of the role of ethylene in
olefin metathesis
(Angew. Chem. Int. Ed. 2020, 59, 22324.
DOI: 10.1002/anie.202010205).
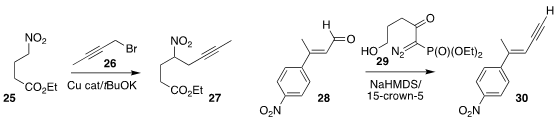
Donald A. Watson of the University of Delaware devised conditions for the
preparation of the nitro alkyne 27 by the
alkylation of the nitroalkane
25 with the propargylic bromide 26
(Org. Lett. 2020, 22, 8106.
DOI: 10.1021/acs.orglett.0c03061).
Yikang Wu of the Shanghai Insitute of Organic Chemistry developed the diazophosphonate 29, a
version of the Ohira-Bestmann reagent
that does not require nucleophilic added alkoxide
(Synlett 2009, 3037.
DOI: 10.1055/s-0029-1218010).
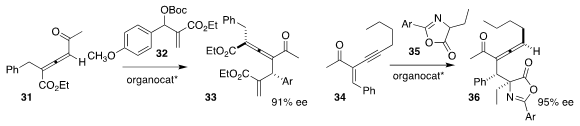
Hongchao Guo of China Agricultural University used a cinchona
alkaloid-derived catalyst to mediate the coupling of 32 with the racemic allene
31, leading to the allene 33 in high ee
(Angew. Chem. Int. Ed. 2020, 59, 19820.
DOI: 10.1002/anie.202009460).
Qian Peng of Nankai University and Xiaoyu Yang of ShanghaiTech University found
that a chiral phosphoric acid effectively directed the construction of the
allene 36 by the addition of 35 to 34
(Nature Commun. 2020, 11, 5527.
DOI: 10.1038/s41467-020-19294-8).
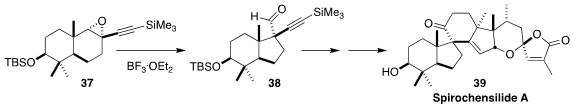
Spirochensilide A (39), isolated from the great Shensi fir, Abies chensiensis,
of central China, Tibet and northern India, significantly reduced physiological
NO production. Jia-Hua Chen and Zhen Yang of Peking University established the
key spiro center of 39 by the rearrangement of the epoxide 37 to the aldehyde
38
(J. Am. Chem. Soc. 2020, 142, 8116.
DOI: 10.1021/jacs.0c02522).