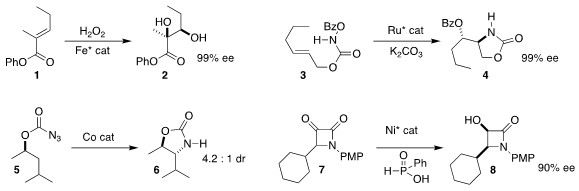
Chi-Ming Che of the University of Hong Kong designed an Fe catalyst that
mediated the enantioselective dihydroxylation of the unsaturated ester
1, delivering the diol 2
(Angew. Chem. Int. 87789-35-3 Formula Ed. 2020, 59, 16561.
DOI: 10.1002/anie.202002866).
Eric Meggers of Philipps-Universität Marburg used a Ru catalyst to convert the N-acyloxy
carbamate 3
to the cyclic carbamate 4
(Org. PMID:34337881 Lett. 2020, 22, 6653.
DOI: 10.1021/acs.orglett.0c02452).
Sukbok Chang of KAIST observed high regioselectivity in the formation of the
cyclic carbamate 6 from the azidoformate 5
(J. Am. 6-Bromo-8-iodoquinolin-2(1H)-one uses Chem. Soc. 2020, 142, 12324.
DOI: 10.1021/jacs.0c04448).
Gen-Qiang Chen and Xumu Zhang of the Southern University of Science and Technology effected the dynamic
kinetic reduction of the racemic α-keto β-lactam 7, leading to the
β-lactam 8 in high ee
(Chem. Commun. 2020, 56, 15557.
DOI: 10.1039/D0CC05599A).
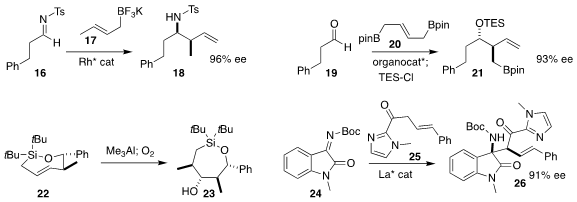
Weidi Cao and Xiaoming Feng of Sichuan University assembled the epoxide 12 by
the triply-convergent coupling of the alkynyl ketone 9, the diazoacetate
10 and the nitrosoarene 11
(Org. Lett. 2020, 22, 6744.
DOI: 10.1021/acs.orglett.0c02108).
Harunobu Mitsunuma and Motomu Kanai of the University of Tokyo achieved high diastereoselectivity in the
addition of commercial 2-butene (14) to citronellal (13) to give 15
(J. Am. Chem. Soc. 2020, 142, 12374.
DOI: 10.1021/jacs.0c04735).
Hsyueh-Liang Wu of the National Taiwan Normal University
prepared the homoallylic sulfonamide
18 by adding the potassium allyl
trifluoroborate 17 to the imine 16
(Org. Lett. 2020, 22, 5675.
DOI: 10.1021/acs.orglett.0c02069).
Ming Chen of Auburn University used a chiral phosphoric acid to mediate the addtion of the
bis-boronate 20 to the aldehyde 19, leading to 21
(Org. Lett. 2020, 22, 8967.
DOI: 10.1021/acs.orglett.0c03366).
K. A. Woerpel of New York University showed that the addition of
trimethylaluminum to the readily-prepared seven-membered ring E alkene 22 and subsequent oxygenation both proceeded with high diastereoselectivity, delivering
the alcohol 23
(Org. Lett. 2020, 22, 7518.
DOI: 10.1021/acs.orglett.0c02711).
Professor Feng and Xiaohua Liu, also of Sichuan University, assembled the α-quaternary amine
26 by adding the acyl imidazole 25 to the imine 24
(Nature Commun. 2020, 11, 3869.
DOI: 10.1038/s41467-020-17681-9).
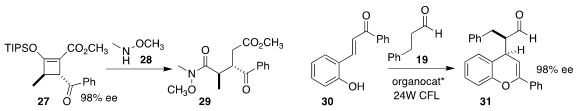
Michael P. Doyle of the University of Texas at San Antonio showed that
included water facilitated the opening of the silyl enol ether 27
(![]()
2017, December 11) with 28, leading to the
Weinreb amide 29
(J. Org. Chem. 2020, 85, 9475.
DOI: 10.1021/acs.joc.0c01176).
Weiqing Xie of Northwest A&F University showed that the photostimulated addition of
aldehyde 19 to the enone 30 could be effectively mediated by a combination of a
chiral phosphoric acid with a chiral imidazolidinone, leading to the flavonoid
precursor 31
(Chem. Commun. 2020, 56, 10018.
DOI: 10.1039/D0CC04424E).
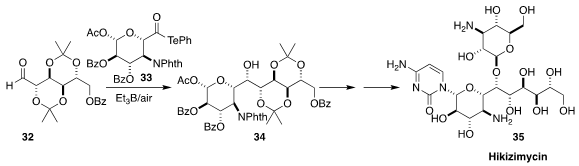
Hikizimycin (35), isolated from a fermentation broth of Streptomyces A-5, was
found to be a powerful antibiotic. A key step in the synthesis of 35 developed by Masayuki Inoue, also of the University of Tokyo, was the free radical
coupling of 32 with 33, leading to 34
(J. Am. Chem. Soc. 2020, 142, 13227.
DOI: 10.1021/jacs.0c06354).