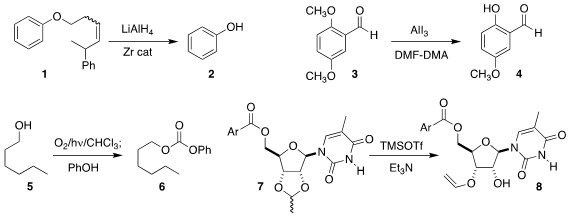
Jan Streuff of the Albert-Ludwigs-Universität Freiburg developed conditions
to remove the alkene-containing alkyl group from 1, leading to 2
(ACS Catal. 2020, 10, 6409.
DOI: 10.1021/acscatal.0c01605).
Dayong Sang and Juan Tian of the Jinchu University of Technology prepared
the salicylaldehyde (4) by selectively demethylating 3
(J. Org. Ethyl 2-amino-1H-imidazole-5-carboxylate Purity Chem. 2020, 85, 6429.
DOI: 10.1021/acs.joc.0c00290).
Akihiko Tsuda of Kobe University devised a protocol for the in situ production of
phosgene, enabling the assembly of the carbonate 6 by the coupling of the alcohol 5 with phenol
(Org. Lett. 2020, 22, 3566.
DOI: 10.1021/acs.orglett.0c01013).
William P. Gallagher of Bristol-Meyers Squibb observed high regioselectivity
in the conversion of the acetal 7 to the vinyl ether 8
(Tetrahedron Lett. PMID:35227773 2020, 61, 151750.
DOI: 10.1016/j.tetlet.2020.151750).
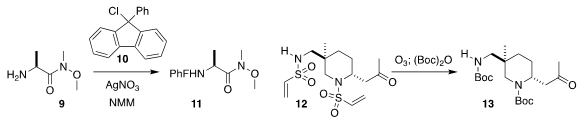
Scott D. Taylor of the University of Waterloo used the chloride 10 in conjunction
with silver nitrate to protect the amine 9 as the 9-phenyl-9-fluorenyl Weinreb amide 11
(J. Org. Chem. 1205671-72-2 custom synthesis 2020, 85, 2068.
DOI: 10.1021/acs.joc.9b02809).
Carlos del Pozo of the University of Valencia introduced the vinyl sulfonamide protecting
group, deprotecting 12 with
ozone, followed by reprotection to give 13
(Chem. Commun. 2020, 56, 1425.
DOI: 10.1039/C9CC09113K).
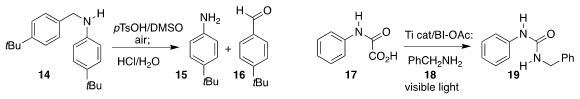
Mitsuru Shindo of Kyushu University oxidized the benzyl amine 14
with DMSO, leading to the aniline 15 and the aldehyde 16
(Chem. Lett. 2020, 49, 191.
DOI: 10.1246/cl.190854).
Liang Wang and Shubai Li of the Changzhou Vocational Institute of Engineering
effected the photooxidation of the oxamic acid 17 to give an activated
intermediate, that was trapped with benzylamine 18 to give the
unsymmetrical urea 19
(Tetrahedron Lett. 2020, 61, 151962.
DOI: 10.1016/j.tetlet.2020.151962).
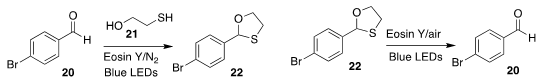
Chin-Fa Lee of the National Chung Hsing University showed that the protection
of the aldehyde 20 with 21 to give 22 could be promoted photochemically
(Eur. J. Org. Chem. 2020, 2542.
DOI: 10.1002/ejoc.202000218).
Conversely, Xingang Xie of Lanzhou University found that the same conditions,
except under air, led to the deprotection of the oxathiolane 22 to 20
(Org. Biomol. Chem. 2020, 18, 288.
DOI: 10.1039/C9OB02517K).
Ramakrishna G. Bhat of the Indian Institute of Science Education and Research
used the same conditions to
deprotect dithianes
(Tetrahedron Lett. 2020, 61, 151407.
DOI: 10.1016/j.tetlet.2019.151407).
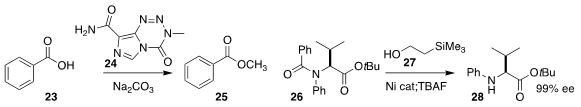
Paul J. Hergenrother of the University of Illinois established that diazomethane could
be generated in situ from 24, to convert the acid 23
to the methyl ester 25
(Angew. Chem. Int. Ed. 2020, 59, 1857.
DOI: 10.1002/anie.201911896).
Neil K. Garg of UCLA showed that 27 in the presence of a Ni catalyst could deprotect
the tertiary amide 26, leading to 28 with the ee maintained
(Org. Lett. 2020, 22, 2833.
DOI: 10.1021/acs.orglett.0c00885).
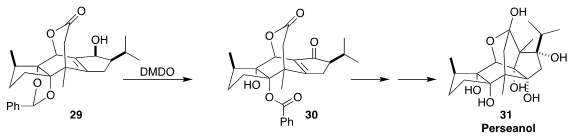
In the course of a synthesis of perseanol (31), Sarah Reisman of Caltech
observed that the oxidation of the allylic alcohol 29 with excess
dimethyldioxirane gave not just the desired enone, but also deprotected the
benzylidene acetal, leading to 30 with significant regioselectivity
(Nature 2019, 573, 563.
DOI: 10.1038/s41586-019-1580-x).