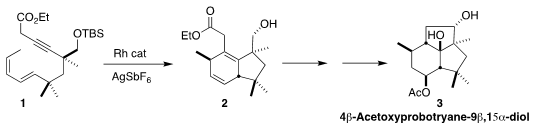
4β-Acetoxyprobotryane-9β,15α-diol (3) was isolated from the wine grape
necrotrophic fungus Botrytis cinerea. En route to 3, Chuang-Chuang Li of the
Southern University of Science and Technology optimized the Rh-catalyzed
cycloaddition of 1 to 2
(J. Am. Chem. 3-Oxoisoindoline-5-carbaldehyde custom synthesis Soc. 2020, 142, 19868,
DOI: 10.1021/jacs.0c10116;
Org. Lett. 2016, 18, 4932,
DOI: 10.1021/acs.orglett.6b02414).
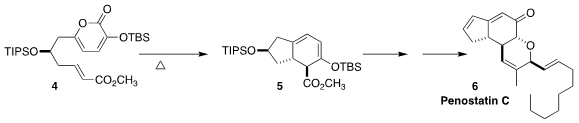
Penostatin C (6), isolated from the entomogenous fungus Isaria tenuipes, is a
0.37 µM inhibitor of protein tyrosine phosphatase 1B. Rongbiao Tong of the Hong
Kong University of Science and Technology assembled the central core 5 of 6 by
the intramolecular Diels-Alder cyclization of 4 followed by spontaneous
decarboxylation
(Org. PMID:34235739 Lett. 2020, 22, 5074.
DOI: 10.1021/acs.orglett.0c01649). 14544-47-9 manufacturer
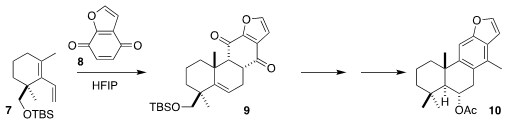
Sucutinirane C (10) was isolated from the South American medicinal plant
Bowdichia nitada. Related compounds have shown selective cytotoxicity against
cancer cells with increased Hedgehog (Hh) signaling levels. Emmanuel N. Pitsinos
of the National Center of Scientific Research "Demokritos" observed high
diastereo- and regioselectivity in the Diels-Alder addition of the quinone 8 to
the diene 7, leading to 9
(Eur. J. Org. Chem. 2020, 4730.
DOI: 10.1002/ejoc.202000724).
Glaucocalyxin A (13), isolated from the Japanese mint Rabdosia umbrosa,
ameliorates myocardial ischemia reperfusion injury. Yanxing Jia of Peking
University assembled 12, having all the skeletal stereogenic centers of 13, by
the unactivated intramolecular Diels-Alder cyclization of 11
(Angew. Chem. Int. Ed. 2020, 59, 15195.
DOI: 10.1002/anie.202005932).
PF-1018 (16) was isolated from a fungal strain identified as Humicola sp. 1018
using a screen against the diamond back moth Plutella xylostella. Dirk Trauner
of NYU prepared 16 via the designed 8π electrocyclization of 14, followed by in
situ Diels-Alder cycloaddition to 15
(Angew. Chem. Int. Ed. 2020, 59, 5263.
DOI: 10.1002/anie.201912452).
Sorensen was the first to develop the intramolecular Diels-Alder approach to
abyssomycin C (19). Veroniki P. Vidali, also of NCSR "Demokritos", significantly
improved on this approach, effecting the oxidation and in situ cyclization of 17 to 18
(Eur. J. Org. Chem. 2020, 4547.
DOI: 10.1002/ejoc.202000671).