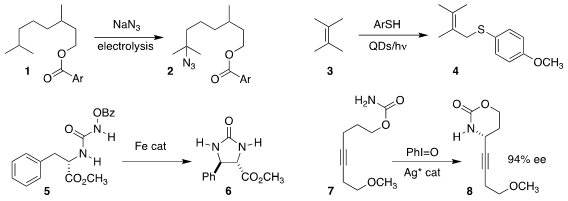
Lutz Ackermann of the Georg-August-Universität Göttingen effected
azidation of 1
selectively at the methine distal to the electron-withdrawing ester, leading to 2
(Chem. Sci. 2021, 12, 2890.
DOI: 10.1039/D0SC05924B).
Li-Zhu Wu of the University of Chinese Academy of Sciences used quantum dots to
promote the preparation of 4 by the
allylic thiolation of the alkene 3
(Angew. PMID:26895888 1403257-80-6 manufacturer Chem. Int. Ed. 2021, 60, 11779.
DOI: 10.1002/anie.202101947).
Eric Meggers of the Philipps-Universität Marburg observed high diastereoselectivity
in the cyclization of the N-benzoyloxyurea 5 to the
imidazolidin-2-one 6
(Angew. Chem. Int. Bromo-PEG2-C2-azide Chemscene Ed. 2021, 60, 6314,
DOI: 10.1002/anie.202013687;
Chem. Commun. 2020, 56, 7714,
DOI: 10.1039/D0CC03280H).
Jennifer M. Schomaker of the University of Wisconsin achieved both high enantioselectivity
and preference for six-membered ring formation in the cyclization of the
carbamate 7 to 8
(J. Am. Chem. Soc. 2020, 142, 12930.
DOI: 10.1021/jacs.0c05726).
Tatsuhiko Yoshino and Shigeki Matsunaga of Hokkaido University reported a parallel investigation
(Nature Catal. 2020, 3, 851.
DOI: 10.1038/s41929-020-00513-w).
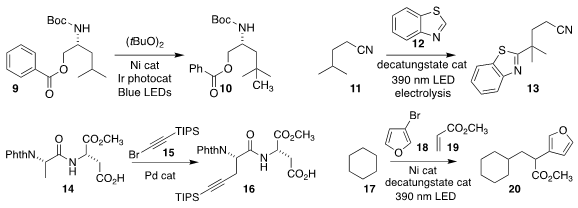
The power of the C-H methylation protocol developed by Shannon S. Stahl, also
of the University of Wisconsin, is demonstrated by the conversion of the
leucine-derived isopropyl group of 9 to the t-butyl group of 10
(Science 2021, 372, 398.
DOI: 10.1126/science.abh2623).
Davide Ravelli of the University of Pavia used a decatungstate catalyst to mediate the coupling
of the methine of 11 with benzothiazole 12, leading to 13
(Chem. Commun. 2021, 57, 4424.
DOI: 10.1039/D1CC01012C).
Yiyi Weng of the Zhejiang University of Technology used a Pd catalyst to assemble
the alkynylated peptide 16 by the coupling of the bromoalkyne 15
selectively with the methyl group of the peptide 14
(ACS Catal. 2021, 11, 7401.
DOI: 10.1021/acscatal.1c01417).
Wangqing Kong of Wuhan University devised the
three-component coupling of cyclohexane 17, the bromofuran 18 and methyl
acrylate 19 to give the ester 20
(Angew. Chem. Int. Ed. 2021, 60, 7405.
DOI: 10.1002/anie.202014632).
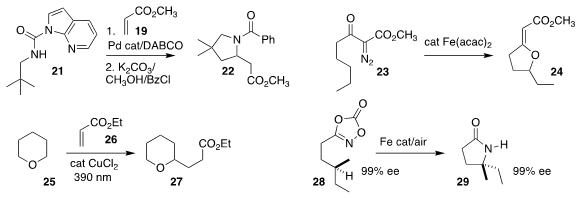
Hongbin Zou of Zhejiang University showed that the selective coupling of
methyl acrylate 19 with one of the methyl groups of 21 followed by deprotection
led to cyclization to the
pyrrolidine 22
(Org. Lett. 2021, 23, 3466.
DOI: 10.1021/acs.orglett.1c00903).
Theodore A. Betley of Harvard University observed that on exposure to an iron catalyst,
the α-diazo β-keto ester 23 cyclized to the
tetrahydrofuran
24
(J. Am. Chem. Soc. 2021, 143, 7480.
DOI: 10.1021/jacs.1c02074).
Tomislav Rovis of Columbia University showed that
tetrahydropyran
25 could be added in a conjugate sense to ethyl acrylate 26, to give 27
(J. Am. Chem. Soc. 2021, 143, 2729.
DOI: 10.1021/jacs.1c00687).
Sukbok Chang of KAIST showed that the cyclization of the dioxazolone 28 to the
lactam 29 proceeded with retention
of absolute configuration
(Angew. Chem. Int. Ed. 2021, 60, 2909.
DOI: 10.1002/anie.202013499).
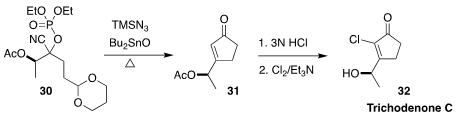
Trichodenone C (32), isolated from the fungus Trichoderma harzianum, showed
significant cytotoxic activity. Shinya Harusawa of the Osaka University of
Pharmaceutical Sciences demonstrated that in the presence of the sideproducts
from the generation of the alkylidene carbene from the cyanophosphate, and
subsequent intramolecular C-H insertion, the product ketal was hydrolyzed,
leading directly to the enone 31. Hydrolysis of the acetate followed by
chlorination then led to 32
(Tetrahedron 2021, 82, 131914.
DOI: 10.1016/j.tet.2020.131914).