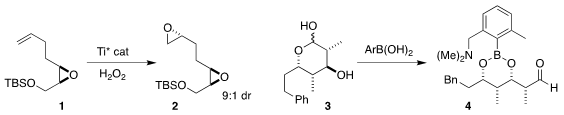
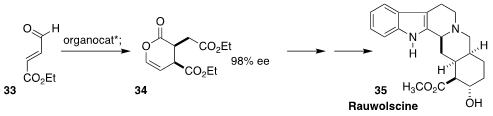David R. Williams of Indiana University showed that the enantioselective
Katsuki
epoxidation of 1 delivered 2
(Org. Lett. 2020, 22, 4118.
DOI: 10.1021/acs.orglett.0c01177).
Harunobu Mitsunama and Motomu Kanai of the University of Tokyo devised a boronic acid
that opened the lactol 3, revealing the aldehyde 4
(Org. Biomol. 5-Bromo-6-fluoro-2-methyl-2h-indazole Chemscene 4-Chloro-2-butenoic acid custom synthesis Chem. 2019, 17, 6562.
DOI: 10.1039/C9OB01263J).
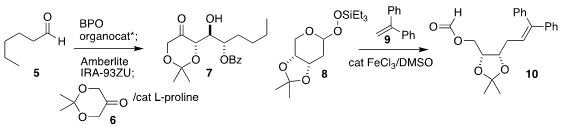
Taichi Kano and Keiji Maruoka of Kyoto University showed that filtration of
the product from the benzoylation of 5 through a weakly basic ion exchange resin
enabled subsequent aldol
reaction with the ketone 6, leading to 7
(Eur. J. Org. Chem. 2020, 2028.
DOI: 10.1002/ejoc.202000073).
Professor Maruoka also devised the assembly of 10 by the
coupling of the peroxide 8 with 9
(Chem. Asian. J. PMID:24059181 2020, 15, 573.
DOI: 10.1002/asia.201901695).
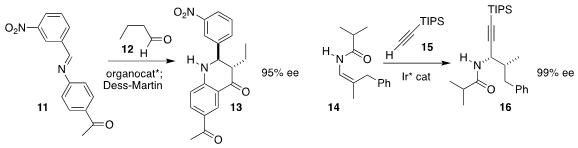
Xiao-Feng Xiong of Sun Yat-sen University used the Hayashi catalyst to
mediate the combination of 11 with 12 leading, after oxidation
with DMP, to 13
(Org. Lett. 2020, 22, 1858.
DOI: 10.1021/acs.orglett.0c00206).
Bi-Jie Li of Tsignhua University established the vicinal
stereogenic centers of 16 by combining 14 with 15
(Angew. Chem. Int. Ed. 2020, 59, 6874.
DOI: 10.1002/anie.201916088).
Chandrakumar Appayee of the Indian Institute of Techology Gandhinagar
also used the Hayashi catalyst to dimerize the ester 17 to the
lactam 18
(Org. Lett. 2020, 22, 4355.
DOI: 10.1021/acs.orglett.0c01375).
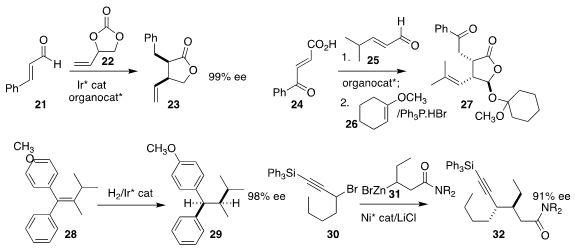
Ilan Marek of Technion prepared 20 by opening the
cyclopropane 19
(J. Am. Chem. Soc. 2020, 142, 5543,
DOI: 10.1021/jacs.0c01133; 7710,
DOI: 10.1021/jacs.0c03380).
Frank Glorius of the Westfälische Wilhelms-Universität Münster combined Ir
catalysis and an NHC organocatalyst to assemble the
lactone 23 by coupling
21 with 22
(Nature Catal. 2020, 3, 48.
DOI: 10.1038/s41929-019-0387-3).
Jun-Bing Lin and Shu-Yu Zhang of Shanghai
Jiao Tong University also used the Hayashi catalyst to mediate the combination
of 24 with 25, leading after protection with 26 to the lactol
27
(Org. Chem. Front. 2020, 7, 571.
DOI: 10.1039/C9QO01367A).
Raphael Bigler of Roche Basel and Haiming Zhang and
Francis Gosselin of Genentech developed the preparation of 29 by the
hydrogenation of the tetrasubstituted alkene 28
(Angew. Chem. Int. Ed. 2020, 59, 2844.
DOI: 10.1002/anie.201912640).
Gregory C. Fu of Caltech effected the coupling of two racemic starting
materials, 30 and 31, to give 32
(Science 2020, 367, 559.
DOI: 10.1126/science.aaz3855).
Rauwolscine (35), isolated from the evergreen tropical "devil peppers" of the
genus Rauvolfia, acts predominantly as a α2-adrenergic
receptor antagonist. Karl A. Scheidt of Northwestern University developed a
general route to such yohimbine alkaloids based on the gram-scale NHC-catalyzed
enantioselective dimerization of 33 to 34
(J. Am. Chem. Soc. 2020, 142, 2187,
DOI: 10.1021/jacs.9b12319;
Chem. Eur. J. 2020, 26, 5794,
DOI: 10.1002/chem.202000747).