The radical chemistry associated with organosilanes has been the subject of my recent book (Chatgilialoglu C. Organosilanes in Radical Chemistry; Wiley: Chichester, 2004). PMID:24605203 In particular, the use of(TMS)3SiH in organic synthesis has been treated in some details with numerous examples. Starting from this month, I will describe some more recent examples of the use of (TMS)3SiH in organic synthesis that are not included in my book. Formula of 1446002-37-4
A radical carboxyarylation approach is introduced by Sherburn and coworkers in two communications as the key step in the total synthesis of several biologically important natural products (J. Am. Chem. Soc. Methyltrioxorhenium(VII) Price 2003, 125, 12108,DOI: 10.1021/ja0376588; Org. Lett. 2004, 6, 1345,DOI: 10.1021/ol049878b). Treatment of thiocarbonate derivatives 1 (R = Me or TBS) with 1.1 equiv of (TMS)3SiH in refluxing benzene and in the presence of AIBN (0.4 equiv added over 6 h) as radical initiator, produced compound 2 in 44% yield. This remarkable transformation resulted from a radical cascade, involving (TMS)3Si. radical addition to thiocarbonyl function (1->3), 5-exo cyclization (3->4) and intramolecular 1,5-ipso substitution (4->5) with the final ejection of (TMS)3SiS. radical.
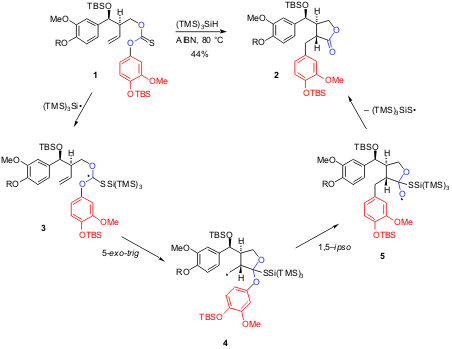
As a personal mechanistic remark, I suggest that the ejected thiyl radical undergoes a fast 1,2-migration of silyl group from silicon to sulfur (Reaction 1), affording a new silyl radical that either reacts with (TMS)3SiH (Reaction 2) completing the reaction cycle, or replaces the (TMS)3Si. radical in the above described reaction sequence.
![]()
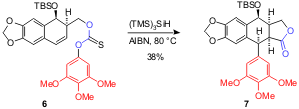
This effective route has also been applied to structurally analogous thiocarbonate 6 that affords 7 in 38% yield. Although the reaction sequence is the same, it is worth underlining that the resulting carbon-centered radical from the 5-exo cyclization is a benzylic-type, which is still reactive towards the 1,5-ipso substitution.