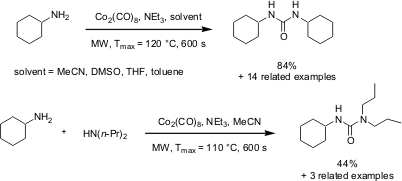
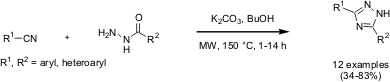Mats Larhed and co-workers from Uppsala University (Tetrahedron Lett. 5-Hydroxymethylfurfural Chemscene 2005, 46, 3335. DOI: 10.1016/j.tetlet.2005.03.076)have reported on a novel and very fast gas-free carbonylation method for the preparation of ureas starting from primary amines. 4-(Methylsulfinyl)aniline site Under high intensity microwave heating with an in situ generation of intermediate isocyanates from Co2(CO)8, amines have been converted to the corresponding ureas within 10 s of reaction times. This method has facilitated the preparation of symmetrical and unsymmetrical ureas in good to moderate yields respectively. PMID:25959043
One Step Synthesis of 3,5-Disubstituted 1,2,4-Triazoles
A recent publication by Kap-Sun Yeung and co-workers from Bristol-Myers Squibb (Tetrahedron Lett. 2005, 46, 3429. DOI: 10.1016/j.tetlet.2005.02.167)describes a convenient and efficient one step, base-catalyzed synthesis of 3,5-disubstituted 1,2,4-triazoles by the condensation of a nitrile and a hydrazide. Under the reaction conditions, a diverse range of functionality and heterocycles are tolerated. The reactivity of the nitrile partner is relatively insensitive to electronic effects.
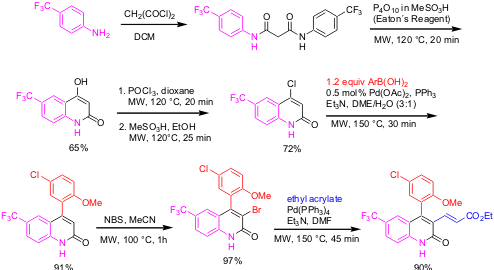
Multistep Synthesis of Functionalized 4-Arylquinolin-2(1H)-ones
An efficient multistep synthesis of biologically active 4-aryl-3-alkenyl-substituted quinolin-2-(1H)-ones has been demonstrated by our group (J. Org. Chem. 2005, 70, 3864, DOI: 10.1021/jo0502549). All the synthetic transformations (six steps) required for the synthesis of the desired target quinolin-2-(1H)-ones have been carried out under controlled microwave heating. The key steps in the synthesis include a sequential Pd catalyzed Suzuki/Heck scaffold decoration method to allow introduction of substituents late in the synthetic scheme and facilitate preparation of 2(1H)-quinolinone analogues with a greater diversity for potentially generating combinatorial libraries.
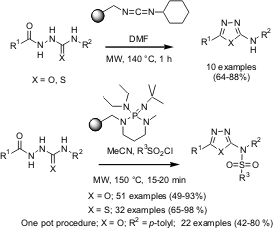
Preparation of 2-Aminosulfonamide-1,3,4-oxadiazoles using Polymer-Supported Reagents and Microwave Heating
Steven Ley and co-workers from the University of Cambridge (Tetrahedron2005, 61, 5323.DOI: 10.1016/j.tet.2005.03.062)have reported on a symbiotic combination of solid-supported reagents and microwave-assisted organic synthesis to rapidly generate compound libraries of 5-substituted-2-amino-1,3,4-oxadiazoles and their correspondingthiadiazole analogues. A one-pot preparation of the 2-aminosulfonylated analogues through a three component coupling of an acylhydrazine, an isocyanate and sulfonyl chloride promoted by a polymer-supported phosphazine base (PS-BEMP) under microwave heating is also described.